Canadian Aquaculture R&D Review 2019
Table of contents
- Introduction
- Finfish: Freshwater
- Finfish: Salmon
- Sea Lice
- Fish Health
- Environmental interactions
- Shellfish: Mussels
- Shellfish: Oysters
- Shellfish: Other
- Miscellaneous
- Glossary
Introduction
Welcome to the eighth edition of the biennial Canadian Aquaculture R&D Review. The review is an ongoing compendium of the aquaculture research and development projects that have been underway over the past two years from all across Canada, whether they are undertaken by researchers from academia, government labs, or other research organisations. The review contains over 150 project descriptions detailing an impressive array of topics, disciplines, species, and geography. Projects include marine and freshwater species with topics ranging from finfish and shellfish health, seaweeds, production, husbandry technology, nutrition, and environmental interactions to name a few.
This is the fifth issue of the review that has been produced by Fisheries and Oceans Canada (DFO) in partnership with the Aquaculture Association of Canada (AAC). This partnership is highly relevant and mutually beneficial to our respective roles in the area of science communication and knowledge translation and mobilisation at both the AAC and DFO. This collaboration has allowed us to produce this 2019 edition as an AAC Special Publication, which is an accessible, electronic citation. Digital versions of this document are also available on both the DFO and AAC websites.
Aquaculture continues to be an important and growing sector of the seafood industry in Canada, as well as globally. As aquaculture continues to grow, the role of science in supporting the sustainable management, regulation, and responsible development of this sector is more crucial than ever. This is coupled with the growing need for healthy and secure seafood products, while ensuring that it occurs in an environmentally responsible manner.
The AAC wants to profile advances in aquaculture research in Canada and provide this information to its members for an expanded dialogue on present and future challenges and opportunities for the industry. As such, this publication falls within the AAC’s mandate of disseminating knowledge and furthering education, and we hope it will continue to be of interest to a wide audience. Likewise, DFO has a mandate to enable the sustainable development of Canada’s aquatic resources, including aquaculture, and to provide access to information on its scientific activities underway within the department and elsewhere in Canada. Publication of ongoing aquaculture research in the Canadian Aquaculture R&D Review contributes towards achieving our shared mandates and to reach out to the science community, interested partners and stakeholders, and the public. Additionally, the publication serves to increase the understanding and breadth of scientific activities underway and encouraging development of collaborations, synergies, and coordination of future activities. Communication and analysis of scientific knowledge is also increasingly pertinent in ensuring a robust evidence-based approach to decision making and regulation of the aquaculture industry, which contributes to improved social understanding, acceptability and public confidence and trust.
We would like to take the opportunity to recognize and thank several people who contributed significantly to the production of this Review. Véronique Boucher Lalonde, Emily Ryall, and Tricia Gheorghe, all with DFO, were instrumental in the overall coordination of this project and in seeing it through to completion, from beginning to end. Dan McPhee, Megan Otu, Lily Weber, Tara Donaghy, Katherine Shepherd, and Zeina El-Aaraj (all with DFO) variously assisted in several aspects of this project. We would also like to thank the AAC office staff (Catriona McLanaghan) and Kim Gill, Chair of the AAC Publications Committee, and her committee, for their support.
G. Jay Parsons, PhD
Ecosystems and Oceans Science Sector
Fisheries and Oceans Canada
Joanne Liutkus
President
Aquaculture Association of Canada
Canadian Aquaculture R&D Review 2019
AAC Special Publication #26
ISBN: 978-0-9881415-9-9
Editors: Tricia Gheorghe, Véronique Boucher Lalonde, Emily Ryall and G. Jay Parsons
Cited as: T Gheorghe, V Boucher Lalonde, E Ryall, and GJ Parsons (eds). Canadian Aquaculture R&D Review 2019. Aquaculture Association of Canada Special Publication 26 (2019)
Finfish: Freshwater
Lake Sturgeon husbandry
Lake Sturgeon have been a long-standing traditional species for subsistence fishing by many Indigenous communities in Ontario. This species was listed as one of ’special concern’ when the Ontario Endangered Species Act took effect in 2008. This research builds on existing knowledge to enhance our ability to produce Lake Sturgeon, with potential applications for stocking into public waters to support provincial fisheries management objectives and to improve husbandry techniques for the commercial aquaculture industry.
Researchers from Wilfrid Laurier University are working together with faculty and staff at the Alma Aquaculture Research Station (AARS) to establish a population of Lake Sturgeon (Acipenser fulvescens) to investigate modes of lampricide toxicity (3-trifluoromethyl-4-nitrophenol) in non-target fishes.
To establish a population at the AARS, wild sturgeon eggs were collected in Northern Ontario by Sustainable Sturgeon Culture in the spring of 2016, 2017 and 2018. Efforts have focused on finding an appropriate early rearing diet and investigating the effects of temperature on growth. The results of this research indicate that early survival (from hatch to ≤0.49 g) is improved by co-feeding both live artemia and a commercial marine diet (e.g., GEMMA Micro from Skretting) immediately after hatch. By 0.5 g, sturgeons are co-fed with blood worm and a commercial diet. Preliminary results indicate a size threshold above which fish will continue to grow even when moved into cooler water temperatures (from 15°C to 8.5°C). Animals transferred to 8.5°C earlier than 6 months post-hatch exhibit a reduction in feeding response and poor growth.
The knowledge that has been developed related to the husbandry of Lake Sturgeon has the potential to inform current rehabilitation initiatives and future interest in sturgeon aquaculture practices.
Date: May 2016 – Dec. 2020
Funded by: Great Lakes Fishery Commission
Co-funded by: University of Guelph
Project Leader: Michael Wilkie (WLU)
Project Team: Oana Birceanu (WLU)
Collaborators: Richard Moccia, Marcia Chiasson, Michael Burke (AARS, U Guelph)
Contact: aars@uoguelph.ca
Website: http://animalbiosciences.uoguelph.ca/aquacentre/aars/aars.html

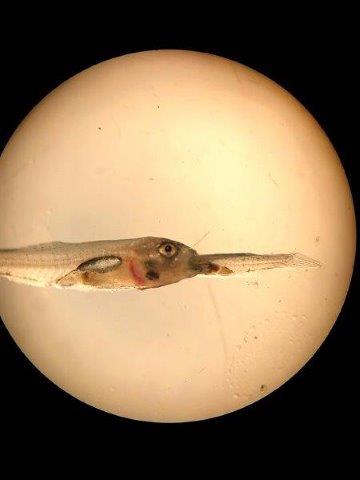
Hatched on May 25, 2017 from wild caught Lake Sturgeon eggs, the fish on top was grown in 15°C water for 11 months, prior to being transferred to 8.5°C . The fish on the bottom was grown in 15°C for 6 months, prior to being transferred to 8.5°C.
Photo: Alma Aquaculture Research Station
Large-scale cyclic restriction-refeeding assays in Salvelinus alpinus, the Hybrid S. alpinus Fraser x S. fontinalis intended for Table Use and S. fontinalis for seeding: maximization and characterization of compensatory growth and description of environmental impacts
Brook Trout (S. fontinalis) and Arctic Charr (S. alpinus) demonstrate excellent tolerance to prolonged feeding restriction in winter during experiments. When returning to a normal feeding, accelerated growth is expressed and allows the attainment of a weight similar to that of a control group that will have eaten to satiety throughout this period. This phenomenon is known as compensatory growth.
The Quebec Arctic Charr Research Group, in partnership with Aquaculture Gaspésie Inc. and Monts-de-Bellechasse Fish Farm Inc., is now proposing a pilot-scale study to introduce feeding restrictions in the commercial production cycle of S. alpinus (Nauyuk), the S. alpinus Fraser x S. fontinalis Baldwin hybrid, and the S. fontinalis Baldwin hybrid for table and seeding purposes. This study aims to demonstrate in a commercial situation (commercial basins and high biomass) that, by imposing a cyclic diet of dietary restriction-refeeding, producers will be able to reduce: 1) the costs related to food and labour, and 2) the organic load discharged into the effluents, with no negative effects on productivity. For farmed fish and sport fish (S. fontinalis), the pre-seeding period of feed restriction should have a positive effect on fishing success by further stimulating the fish to seize the bait.
On the other hand, since it has already been demonstrated that the inclusion of nucleotides in the food facilitates the growth recovery in fish, our study also proposes, in parallel, to analyse the effect of the introduction of a nucleotide supplemented feed on S. alpinus (Nauyuk) growth recovery to reduce the time required to achieve complete compensatory growth and weight similar to, or greater than, the control group (i.e., fed to satiety).
Finally, Arctic Charr in an aquaculture context is often characterized by the rapid emergence of a hierarchy of dominance. Therefore, a rigorous monitoring of the hierarchical stability during treatments and during the recharge phase will be investigated.
Date: Nov. 2018 – Sep. 2021
Funded by: Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) – Innovamer Program; Montreal Biodome
Co-funded by: Aquaculture Gaspésie inc.; Pisciculture des Monts-de-Bellechasse inc.
Project Leader: Nathalie R. Le François (Montreal Biodome)
Project Team: Simon G. Lamarre (U Moncton); Pierre U. Blier, Ariane Savoie (UQAR); Charles D. Johnson (Montreal Biodome)
Collaborators: Francis Dupuis (Aquaculture Gaspésie inc.); Moïse Cantin (Pisciculture des Monts-de-Bellechasse inc.)
Contact: NLe_Francois@ville.montreal.qc.ca
Impairment of phosphorus absorption in Rainbow Trout: Physiological adaptations to phosphorus deficiency
The proposed project challenges the view that the only source of phosphorus (P) available to freshwater fish is their food. This idea has been largely accepted as P concentrations in the natural environment of freshwater fish are relatively low (<0.1 ppm P in water), rendering them unable to absorb appreciable amounts of P from the outside environment.
Two experiments were carried out: a closed circuit experiment (2017) and another one with the two circuits (open and closed) combined (2018). Preliminary results show that the deficient fish (scale ash = 25%) in a closed circuit absorb some of the inorganic phosphorus in the breeding ponds during the accumulation test of this element. Meanwhile, fish with the same scale ash as open-circuit deficient fish and sufficient fish (scale ash = 27–28%), regardless of the circuit, show no ability to absorb phosphorus in the water basins. These observations indicate the presence of a signal inducing this adaptation in the closed circuit. The signal could be related to the accumulation of inorganic phosphorus in the basins during the cycle. Data from the contention testing of fish may confirm these observations. Expression evaluation, by qPCR, of proteins involved in transmembrane phosphorus transport (I-NaPi, PC-NaPi and PiUS) and immunohistochemistry (Abs anti-NaPi) in the gills and skin could validate the hypothesis that this adaptation would go through the overexpression of these proteins.
The results of this project will contribute significantly to the understanding of phenotypic plasticity in fish. Our work will advance knowledge of the impact of a physiological disturbance (i.e., nutritional deficiency) and will identify new strategies for homeostasis.
Date: Jan. 2013 – Mar. 2018
Funded by: Natural Sciences and Engineering Research Council (NSERC)–Discovery Program
Project Leader: Grant W. Vandenberg (U Laval)
Project Team: Waly N. Ndiaye, Marie-Hélène Deschamps, Émilie Proulx (U Laval)
Contact: grant.vandenberg@fsaa.ulaval.ca
Website: http://www.vrrc.ulaval.ca/fileadmin/ulaval_ca/Images/recherche/bd/chercheur/fiche/424160.html

A Rainbow Trout, deficient in phosphorus, is immobilized to measure the specific absorption of the gills.
Photo: Émilie Proulx (U Laval)
Study of apparent digestibility of black soldier fly larvae (Hermetia illucens) meal in Rainbow Trout
With the expansion of aquaculture and the rising price of fish meal and soybean meal, the main sources of protein in fish diets, it is necessary to find alternative foods. Insect meal from the “overcycling” of residual organic matter is a source of high quality eco-friendly protein and energy that has great potential for the aquaculture industry. However, the digestibility of nutrients from insect meal can be influenced by a multitude of factors during production and, in particular, by the stage of development of the insect during harvest.
The purpose of this project is to characterize the effects of the use of black soldier fly larvae of different larval stages (larvae, pre-pupae) on nutrient digestibility in juvenile Rainbow Trout. To do this, in vitro assays are performed to measure the presence of anti-nutritional factors (inhibition of protease activity in intestinal homogenates), and nutritional tests will be conducted to measure the apparent digestibility of nutrients.
Our results will help to identify the anti-nutritional factors present in black soldier flies and find ways to optimize the processing of the product. Ultimately, we will be able to develop first tools for the formulation of insect-based diets for Rainbow Trout.
Date: Jan. 2018 – Dec. 2019
Funded by: Ministère de l’agriculture, des pêcheries et de l’alimentation du Québec (MAPAQ) – Innov’Action agroalimentaires Program
Co-funded by: Formation agricole pour la sécurité alimentaire au Mali (FASAM)
Project Leader: Grant W. Vandenberg (U Laval)
Project Team: Bakary Diarra, Marie-Hélène Deschamps, Linda Saucier, Cristina Ratti, Alain Doyen, Lucie Beaulieu, Marie-Pierre Létourneau Montminy (U Laval); Marie-Pier Aubin, Charles Lavigne (QAIC)
Contact: Grant.vandenberg@fsaa.ulaval.ca
Development of a Rainbow Trout stock performance improvement strategy for commercial production in Nova Scotia, Canada
Presently, the Rainbow Trout industry in Nova Scotia has access to a limited number of approved sources of eyed eggs for production. The primary source is a single supplier in the United States with the ability to supply all-female, as well as triploid eggs, and currently meets the fish health and biosecurity requirements established by Canadian regulators. There is an opportunity to engage additional suppliers to ensure business continuity, but such alternatives must demonstrate comparable and predictable performance of commercially relevant traits in Nova Scotia.
Member Rainbow Trout producers of the Aquaculture Association of Nova Scotia recognize the need to assess its presently available genetics and egg supplier situation. This project met this need by developing a plan (methods, facilities and timeframe) to benchmark the performance of commercially important traits across a broad representation of possible sources of eyed eggs, reviewing best practices for farming Rainbow Trout in Nova Scotia, and assessing options to develop a local broodstock program that meets the stated breeding goals of Nova Scotia producers. The broodstock program component followed an established Huntsman method to:
- Consult with industry partners to establish breeding goals, rank company- and sector-specific traits of interest and foster open dialogue;
- Review the state of knowledge of Rainbow Trout heritabilities, genetic and phenotypic correlations, and molecular markers pertaining to specific traits of interest identified by the commercial producers;
- Visit operational facilities (primarily land-based production and processing facilities) to assess capacity within the Nova Scotia Rainbow Trout production ecosystem to support aspects of a comprehensive broodstock program; and
- Recommend potential broodstock program models, including various trait-specific challenges and data collection approaches, which will offer the greatest likelihood for success, taking into account all of the pertinent information attained from the previous steps.
This project will establish the framework to stabilize and increase Rainbow Trout production in Nova Scotia, from a broodstock management perspective. An overall strategy will be offered to the industry, including timelines, possible funding opportunities, identified private sector and academic collaborators, and, importantly, reasonable anticipated outcomes to their farming operations and financial well-being resulting from a genetic selection broodstock program.
Date: Sep. 2018 – Jan. 2019
Funded by: Genome Atlantic
Co-Funded by: Aquaculture Association of Nova Scotia (AANS)
Project Leader: Amber Garber (HMSC)
Project Team: Farhad Amini, Chris Bridger (HMSC); Christophe Herbinger (Dalhousie U)
Collaborators: Isabelle Tremblay (AANS)
Contact: amber.garber@huntsmanmarine.ca
Website: Huntsmanmarine.ca
Striped Bass aquaculture research & development in Nova Scotia
The strong market demand for Striped Bass (Morone saxatilis) offers a great opportunity for intensive aquaculture. Our goal is to help establish a sustainable land-based Striped Bass industry in the Maritimes, providing valuable diversity to the finfish sector. Three MSc projects are ongoing in Nova Scotia to investigate critical aspects in the culture of Striped Bass.
The first project is investigating parameters that affect cannibalism among Striped Bass larvae. The incidence of cannibalism, survival and growth were significantly affected by stocking density of both Striped Bass larvae and Artemia prey. Under sub-optimal density conditions, cannibals can become 10-fold larger than ’regular’ larvae within 14 days and decimate the population by 50% or more.
The second project assessed grow-out performance in Millbrook First Nations’ recirculation system; Striped Bass grew from 60 to 800 grams in 10 months reared in 23°C freshwater, and achieved an FCR of around 1.1 (Ewos Vita diet).
The third project is assessing Striped Bass juvenile performance in cages suspended in constructed freshwater ponds. Pond-reared bass grow fast from June to September when temperatures exceed 18ºC, but they do not feed in winter (November to April), requiring two summer growing seasons after their transfer to the ponds (at 100 g) to reach market size (1 kg). Hypoxia tolerance in summer is remarkably high. Overwinter mortality is high among under-yearlings less than 100 g in size, but is negligible among yearlings larger than 250 g.
Together, these projects are helping define the scope for Striped Bass aquaculture in Nova Scotia. Establishing a broodstock program and improving egg quality remain substantial challenges.
Date: May 2017 – Sep. 2019
Funded by: Nova Scotia Department of Fisheries and Aquaculture (NSDFA)
Co-Funded by: NSERC; MITACS Canada; NS Graduate Scholarship Program, NS Business Inc. (Productivity and Innovation Voucher Program)
Project Leader: Jim Duston (Dalhousie U)
Project Team: Kare Tonning, Shanwei Qiu, Desiree Roberts (Dalhousie U)
Collaborators: M. Cameron (North River Fish Farms Ltd.); M. Spencer (Millbrook First Nations)
Contact: jduston@dal.ca
A 16 mm long bass larva consuming a 11 mm long larva
Photo: Shanwei Qiu (Dalhousie U)
A comparison of GIS - and hydrodynamic-based depositional modelling for the purpose of predicting freshwater finfish cage footprints
Aquaculture regulators frequently require the use of a depositional model to accompany new site licence applications, and to support requests for changes to feed quotas or production. Several models to predict the extent and intensity of deposition of solid waste from fish farms and associated environmental impacts have been developed, of which DEPOMOD is the most widely used. However, within freshwater systems, DEPOMOD is not able to predict certain parameters with accuracy, and the production of a DEPOMOD prediction is time consuming. In addition, it requires extensive data collection and analysis using specialized software that must be purchased and licensed. Although other modelling processes exist, such as FVCOM, they too tend to be data intensive and expensive. Therefore, it is worth exploring an alternative and more practical modelling process for freshwater systems.
A modelling approach that incorporates cage movement, while focusing less on the details of hydrodynamic processes, may produce footprint predictions with similar accuracy at less cost to the producer and still meet the need of the regulator. Additionally, if this approach proves promising, then it may become possible in the future to consider how mooring standards could be designed as a way to minimize benthic impacts of deposition.
This project aims to use existing data sets to compare the error of depositional modelling estimated using DEPOMOD to that of a GIS-based model that incorporates cage movement. This information can then be used to develop a new basic computational program that will more accurately estimate the depositional footprint of a cage farm based on feed usage and quality, production size, average current speeds, and bathymetry.
Date: Apr. 2018 – Jun. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Aqua-cage Fisheries Ltd.
Project Leader: Cheryl Podemski (DFO)
Project Team: Jamie Raper (DFO); Gord Cole (Aqua-cage Fisheries Ltd.)
Collaborators: Jamie Hooft (Aqua-cage Fisheries Ltd.)
Contact: cheryl.podemski@dfo-mpo.gc.ca
In situ chelation of phosphorus using microencapsulated aluminum and iron sulfate to bind intestinal phosphorus in Rainbow Trout (Oncorhynchus mykiss)
Excess phosphorus in freshwater ecosystems increases primary production which, left uncontrolled, may lead to eutrophication, accelerating the aging process of receiving water bodies. To limit phosphorus release resulting from feeding fish, we propose to incorporate microencapsulated P-chelating agents into fish diets.
In a first trial, alum (Al2SO4) and ferrous sulfate (FeSO4) were encapsulated by spray-chilling in a hydrogenated lipid matrix. Two practical diets incorporating one of these two chelating elements (6 g kg-1) were fed to fish for five weeks, and P release from resulting feces was compared. In a second trial, a similar approach was used to evaluate the impact of increasing supplementation of encapsulated alum (3, 6, 15 g kg-1 diet). Feces from the fish fed with the diets incorporating alum and ferrous sulfate release had 54% and 38% less phosphorus than those from fish fed with control diets, respectively. The second experiment revealed a negative correlation between the level of encapsulated aluminium sulfate included in the diet and phosphorus released by the feces (y = -1.13x + 10.9; R2 = 0.81). Feces from feed incorporating aluminium sulfate at 0, 3, 6 and 15 g kg-1 released, had 69%, 58%, 43%, and 34% of the fecal P after 14 days, respectively. Fish fed encapsulated aluminium sulfate have similar growth performance and mineral status.
Incorporation of encapsulated P-chelating agents into fish feed may offer an opportunity to manage phosphorus levels from effluents, particularly from fish feces resulting from the Quebec fish farms sector.
Date: Apr. 2014 – Oct. 2018
Funded by: Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) –Innovamer Program
Project Leader: Grant W. Vandenberg (U Laval)
Project Team: Waly N. Ndiaye, Muriel Subirade, Marie-Hélène Deschamps (U Laval)
Collaborators: Yves Comeau (École Polytechnique de Montréal)
Contact: grant.vandenberg@fsaa.ulaval.ca
Novel feed additive analysis for early life stage Rainbow Trout (Oncorhynchus mykiss)
Oreka Solutions is an Ontario-based company that uses innovative components in animal feeds. Oreka is looking at ways to yield better FCR values in addition to improving the health of the fish consuming their products (decreasing mortality rates, increasing resistance to pathogens, and optimizing production cycle times). The proposed research project will evaluate the extent to which feed formulations can produce improvements in FCR and early life stage survivorship for Rainbow Trout living in typical hatchery conditions.
The developed product could reduce the need for antibiotics in aquaculture. As a feed supplement, it will provide the necessary raw materials and ingredients to ignite the metabolism of young fish, thereby facilitating a high functioning immune system which enables the animal to fend off life-threatening bacteria.
Date: Oct. 2018 – Jan. 2019
Funded by: Natural Sciences and Engineering Research Council (NSERC)
Project Leader: Brent Wootton (Fleming College)
Project Team: Jon Carter, Ryan Hill (Fleming College)
Collaborators: Oreka Solutions
Contact: Jon.carter@flemingcollege.ca; ryan.hill@flemingcollege.ca
Website: www.flemingcollege.ca

Preparing feeding trials at Oreka Solutions.
Photo: Jon Carter (Fleming College)
Finfish: Salmon
Migration routes, residence time and survival of juvenile salmon in the Strait of Georgia and the Discovery Islands region
Since 2004, Kintama has been using a large-scale acoustic telemetry array to track salmon smolts during their freshwater and early marine migration. Fish are tracked with a network of acoustic sensors positioned in the Fraser River basin and throughout the greater Salish Sea area. By reconstructing the movements of each individual recorded by the array, it has been possible to estimate survival, residence time and travel rate through the Strait of Georgia (SOG) and as far as the North-Eastern end of Vancouver Island.
Beginning in 2010, Kintama and UBC began collaborating on Chilko Lake sockeye migration studies. They found three major mortality trends: high mortality in the small freshwater tributaries leading to the Fraser, low mortality in the Fraser River mainstream, and higher mortality between the northern SOG and the northern end of Vancouver Island relative to the central SOG. Travel times were typical for migrating salmon in the SOG (~ 1 body length per second), and then increased through the Discovery Islands and Johnstone Strait. Additional receivers deployed in 2015 determined that juvenile sockeye used all possible migration routes through the Discovery Islands.
In 2017 and 2018, Kintama deployed receivers near salmon farms in the Discovery Islands to evaluate fish exposure time to salmon farms. Median travel time through Hoskyn and Okisollo channels was 46 hours. Therefore, exposure time to individual salmon farms was short. Median time within the vicinity of the farms was less than 13 minutes.
This work has been important in further characterizing the migration timing of juvenile salmon species from the freshwater to marine environment. Further, it has provided insight, for the first time, into the duration of time juvenile salmon spend near salmon farms on their outward migration.
Date: Apr. 2014 – Oct. 2018
Funded by: Pacific Salmon Foundation – Salish Sea Marine Survival Program
Co-funded by: Ocean Tracking Network; BC Salmon Farmers Association (BCSFA); Fisheries and Oceans Canada (DFO); Hakai Institute; Natural Sciences and Engineering Research Council of Canada (NSERC)
Project Leader: David Welch, Erin Rechisky (Kintama Research Services Ltd.)
Project Team: Aswea Porter (Kintama); Scott Hinch, Christine Stevenson, Stephen Johnston (UBC)
Collaborators: Kintama; UBC
Contact: Erin.Rechisky@kintama.com; joanne@bcsalmonfarmers.ca
Website: Kintama.com
Hybridization of farmed escaped and wild Atlantic Salmon: So what? An empirical and model-based exploration of the consequences for wild populations throughout the North Atlantic
Interbreeding between wild and escaped farmed salmon has been reported both in Europe and North America and can alter wild population characteristics, eroding local adaptation and causing wild population declines. The resiliency of wild populations to escapees, the recovery time following hybridization, and the efficacy of possible mitigation strategies remain unclear, and hamper management efforts in North America and Europe.
Currently, independent studies are underway across the North Atlantic to explore the genetic consequences of farm-wild interbreeding and the ultimate impact on population stability and persistence. The goal in each case is to quantify the extent and magnitude of genetic impacts due to farmed escaped salmon, directly informing mitigation and risk management strategies.
The overall objective of this international collaborative research, which is under the auspices of the “Galway Aquaculture Working Group”, is to provide the basis for robust scientific advice regarding the genetic impacts of escaped farmed Atlantic Salmon on wild populations both locally (i.e., Newfoundland) and across the North Atlantic. Specifically, this work will directly complement existing studies and will: (1) quantify the magnitude of hybridization among wild and escaped farmed salmon and explore growth, survival and biological differences among wild and hybrid individuals; and (2) develop an international collaboration to explore optimal approaches to model these interactions and the subsequent impact on population productivity and stability. This work will directly foster international collaboration aimed at better understanding and managing the impact of farmed escaped salmon on wild populations throughout the North Atlantic.
Date: Apr. 2016 – Mar. 2019
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Ian Bradbury (DFO)
Project Team: Steven Duffy, Lorraine Hamilton, Carole Grant, Chris Hendry, Brian Dempson, Ross Jones (DFO)
Collaborators:Ian Fleming, Matt Rise (MUN); Kjetil Hindar (NINA); Kevin A. Glover (IMR); Eric Verspoor, Mark Coulson (RLI); Phil McGinnity (UCC); Einar Nielsen (DTU); Kristen Gruenthal (NOAA)
Contact: ian.bradbury@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-NL-11-eng.html

Atlantic Salmon sea cages in southern Newfoundland.
Photo: KÖBB Media / DFO
Use of hydro-acoustic methods to assess the migration timing and distribution of juvenile salmon in Discovery Islands and Johnstone Strait
During their migration to the Northern Pacific, juvenile wild salmon from the Strait of Georgia pass through the Discovery Islands and Lower Johnstone Strait, where salmon farming occurs. This project examined the risk of disease transfer between wild and farmed salmon in this area by studying wild salmon migratory pathways and the duration of their residency in the vicinity of fish farms. The effectiveness of hydro-acoustics as a way of monitoring fish abundance, behaviours, and distribution for extended and continuous periods of time was evaluated.
A two-year dataset was gathered for a site located in Okisollo channel, in the Discovery Islands region near a Cermaq Atlantic Salmon farm. Acoustic monitoring data showed strong agreement with data collected using purse seine and trawl methods. Peak migration occurred from mid-May to mid-July in 2015, and from mid-May to mid-June in 2016. Total abundances (all salmon species confounded) were similar in 2015 and 2016. The acoustic time series provided detailed information on schools structure, density, and depth. Results suggest that high abundances of juvenile salmon are present in Okisollo channel during fish farm active operations. However, to what extent the juvenile salmon interact with the fish farms and the degree of proximity is still unclear, and further research is required to investigate these aspects.
Results generally show that inverted echosounders provide a cost-effective, non-intrusive option for long-term monitoring of wild fish populations in this region.
Date: May 2015 – Jun. 2017
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: BC Salmon Farmers Association (BCSFA)
Project Leader: Stéphane Gauthier (DFO)
Project Team: Stewart Johnson, Marc Trudel, Chrys-Ellen Neville (DFO)
Collaborators: Joanne Liutkus (BCSFA)
Contact: Stephane.Gauthier@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-P-02-eng.html
Acoustic monitoring of wild fish interactions with aquaculture sites — Phase I
The sustainability of the British Columbia salmon aquaculture industry is widely questioned due to concerns related to the potential risk posed by farmed salmon to wild salmon. This project monitored the migratory pathways of wild salmon and the duration of their residency in the vicinity of fish farms.
The project showed that acoustic systems can successfully monitor, in near real-time, populations of juvenile wild salmon in areas utilized by the aquaculture industry. The project was also successful in using imaging sonars to monitor wild fish interactions with aquaculture facilities. These systems provide detailed data on the migration timing, duration, and dynamics of wild salmon populations. Results from this project :
- Showed that juvenile salmon behaviour near aquaculture sites appears to vary in relation to both the diurnal and tidal cycles, as well as fish size;
- Documented the number of juvenile salmon schools interacting with fish farms from May to August, allowing to infer disease transfer potential;
- Provided detailed population monitoring in the Okisollo channel, from which salmon productivity can be estimated.
Data collected as part of this project are needed to assess the sustainability, and inform mitigation measures if needed, of the aquaculture industry around the Discovery Islands in BC.
Date: Aug. 2017 – Aug. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cermaq Canada Ltd.; Marine Harvest Canada Limited
Project Leader: Stéphane Gauthier (DFO)
Project Team: Stewart Johnson, Chrys Neville, Marc Trudel (DFO)
Collaborators: Barry Milligan (Cermaq Canada Ltd.); Sharon DeDominicis (Marine Harvest Canada Limited)
Contact: Stephane.Gauthier@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-P-01-eng.html

Brown’s Bay processing plant.
Photo: Stéphane Gauthier (DFO)
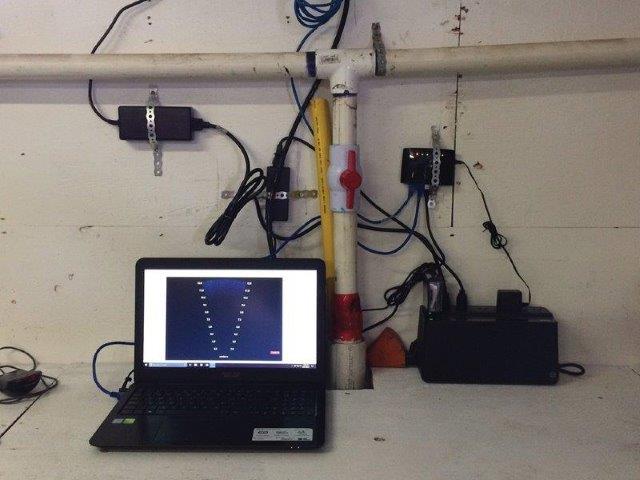
DIDSON recording station.
Photo: Stéphane Gauthier (DFO)

Cermaq Venture Point site.
Photo: Stéphane Gauthier (DFO)

Marine Harvest site in Okisollo.
Photo: Stéphane Gauthier (DFO)
Acoustic monitoring of wild fish interactions with aquaculture sites — Phase II
This project will be a continuation and expansion of a previously funded ACRDP project, which monitored wild fish interactions with Atlantic Salmon farms using a high resolution imaging sonar and multi-frequency echo sounders. For the continuation of the project, experiments will be repeated for two consecutive seasons to further assess the potential variations due to wild salmon juvenile species, ensuring that interactions at fully stocked fish farm facilities throughout the summer migration period are monitored.
To assess the timing and dynamics of wild salmon migration, and interactions of wild fish at aquaculture facilities, the two main objectives of this project are to: 1) monitor activity of wild fish in the direct vicinity of aquaculture sites using high resolution imaging sonar mounted on aquaculture facilities; and 2) continue the monitoring of wild salmon migration in Okisollo channel using moored echo sounders close to the aquaculture facility.
Date: Jun. 2018 – Mar. 2020
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cermaq Canada Ltd.; Marine Harvest Canada
Project Leader: Stéphane Gauthier (DFO)
Project Team: Ben Snow, Stewart Johnson, Chrys-Ellen M. Neville, Marc Trudel, Shani Rousseau (DFO)
Collaborators: Barry Milligan (Cermaq Canada Ltd.); Sharon DeDominicis (Marine Harvest Canada)
Contact: Stephane.Gauthier@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-p-06-eng.html

DIDSON set-up.
Photo: Stéphane Gauthier (DFO)

Moorings before deployment.
Photo: Stéphane Gauthier (DFO)

Cermaq Venture Point.
Photo: Stéphane Gauthier (DFO)

Installing DIDSON.
Photo: Stéphane Gauthier (DFO)
Migration timing and distribution of juvenile salmon in Discovery Islands and Johnstone Strait
During their migration to the Northern Pacific, juvenile wild salmon from the Strait of Georgia (SOG) pass through the Discovery Islands (DI) and Lower Johnstone Strait, where salmon farming occurs. This project evaluated the risk of disease transfer associated with interactions between wild and farmed salmon in this area by studying wild salmon migratory pathways and the duration of their residency in the vicinity of these fish farms.
Data from net-based and hydroacoustic surveys, collected over three years of outmigration (2014-2016), show that juvenile Fraser River Sockeye Salmon enter the SOG over a five to six week period (mid-April – May) and spend five to eight weeks, depending on the year, in the SOG during which they migrate northwards. With exception of the Harrison Lake stock, all stocks of Fraser River Sockeye Salmon co-migrate with other juvenile salmon through the DI over a five to eight week period (mid-May through July), with the majority of the migration occurring over a two to three week period (late May to early June).
In addition, this project is the first to report juvenile Sockeye Salmon carrying infectious hematopoietic necrosis virus (IHNV) in marine waters. It is not known whether these carriers shed virus but, if they do, this may be a significant source of IHNV exposure to farmed salmon.
This project also provided the first estimate of the duration of interactions between farmed salmon and juvenile Sockeye Salmon in the DI, which could then be incorporated in management practices to reduce the potential risks associated with these interactions. Moreover, information from this project has been, and is currently being used in a Risk Assessment of pathogen transfer from farmed salmon to wild Fraser River Sockeye Salmon.
Date: May 2014 – Jun. 2016
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: BC Salmon Farmers Association (BCSFA)
Project Leader: Stewart Johnson (DFO)
Project Team: Stéphane Gauthier, Marc Trudel, Chrys-Ellen Neville (DFO)
Collaborators: Joanne Liutkus (BCSFA)
Contact: Stewart.Johnson@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/P-14-01-001-eng.html
Migration of Atlantic Salmon post-smolts and their interactions with aquaculture in Passamaquoddy Bay, New Brunswick, Canada
We are conducting an acoustic telemetry project in Passamaquoddy Bay, New Brunswick, to provide information on Atlantic Salmon post-smolts, including their migration routes out of the Passamaquoddy Bay, the speed at which they move out of the bay, their survival rates in the estuary and in the ocean, and to determine if there is any interaction with salmon aquaculture.
For this project, we are implanting acoustic tags in salmon smolts that have been reared in substrate ponds at the Mactaquac Biodiversity Facility, and releasing them below the dam in the Magaguadavic Basin. Their migration and survival is monitored with receivers placed in the Magaguadavic Basin and estuary, on either side of the mouth of the estuary, at all the exit points of Passamaquoddy Bay, and at active and inactive salmon aquaculture sites. These receivers will detect if any of the smolts that have been implanted with an acoustic tag are migrating nearby. We are also manually tracking smolts in the bay, and have installed current meters to determine to what extent salmon post-smolts are using currents during their migration at sea.
This research will provide the first estimate of residence time of Atlantic Salmon post-smolts near aquaculture sites in Eastern Canada. Results of this research will be used to inform the risk of disease transfer from Atlantic Salmon aquaculture to wild Atlantic Salmon.
Date: Jan. 2017 – Sep. 2021
Funded by: DFO – Aquaculture Ecosystems Interactions Program (DFO – AEIP)
Co-Funded by: NSERC Strategic Partnership Grant
Project Leader: Marc Trudel (DFO)
Project Team: Brent Wilson, Chris McKindsey, Fred Page (DFO); James Hawkes (NOAA Fisheries); Glenn Crossin (Dalhousie U)
Collaborators: Susan Farquharson (ACFFA); Jason Daniel, Jon Carr (ASF); Fred Whoriskey (Ocean Tracking Network); David Hardie, Ross Jones, John Whitelaw (DFO)
Contact: Marc.Trudel@dfo-mpo.gc.ca

Marc Trudel performing salmon surgery.
Photo: Brent Wilson (DFO)

Marc Trudel deploying receivers.
Photo: Brent Wilson (DFO)

Releasing salmon.
Photo: Brent Wilson (DFO)
Genomic baseline for the quantification of indirect genetic impacts of triploid Atlantic Salmon aquaculture in Placentia Bay, Newfoundland
In 2013, DFO Science evaluated the potential effects of domesticated Norwegian Atlantic Salmon on wild Atlantic Salmon in Newfoundland and recommended the production of all-female sterile triploids to reduce the likelihood of direct genetic effects on wild fish populations. In 2016, an aquaculture expansion in Placentia Bay was approved, involving the production of seven million triploid Norwegian salmon annually. The use of triploid all-female salmon is expected to reduce direct genetic interactions, although the actual magnitude of direct and indirect genetic interactions remains unknown.
This project will develop genomic baseline data to allow future resolution of any direct and indirect genetic impacts. Additionally, this project will develop genomic tools for the identification of escapee Norwegian salmon and hybrids; the genome wide characterization of diversity to allow quantification of changes in wild populations due to changes in the selective landscape (i.e., disease, parasite, competition); and finally the estimation of effective population size of wild populations in Placentia Bay to allow declines in wild population size to be quantified in future. The baseline genomic data produced here will provide unprecedented resolution of direct and indirect genetic impacts due to aquaculture expansion introduced in Placentia Bay and better inform aquaculture management and risk mitigation measures.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO – Aquaculture Ecosystems Interactions Program (DFO - AEIP)
Project Leader: Ian Bradbury (DFO)
Contact: Ian.Bradbury@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-nl-05-eng.html
Fundy Salmon Recovery
Fundy Salmon Recovery is a collaboration of partnering organizations from government, industry, academia and Indigenous communities working together to recover inner Bay of Fundy Atlantic Salmon through their state-of-the-art recovery model. Through collaboration and innovation, their goal is to restore wild Atlantic Salmon populations in the inner Bay of Fundy.
The Fundy Salmon Recovery model works to maximize the early exposure to the wild, resulting in fish that are better at surviving and reproducing. Young salmon are collected in their native rivers and brought to the world’s first wild Atlantic Salmon Marine Conservation Site in Dark Harbour, Grand Manan, managed by Cooke Aquaculture. When mature, the adult salmon are released back to Fundy National Park rivers to spawn naturally.
This model is already producing results; with the release of successfully spawning adults, we are seeing abundant wild juvenile salmon populations throughout the Upper Salmon River in Fundy National Park. After spawning, these adult salmon leave the river and we have seen high numbers of salmon returning to spawn a second and third year. These returns have recently contributed to 29-year high salmon counts in Fundy National Park rivers.
In the fresh water of the Upper Salmon River, ecosystem productivity has significantly improved due to marine nutrients attributed to the presence of high numbers of adult salmon. This research suggests that this increased productivity translates to improved feeding and survival conditions for juvenile salmon that would not exist without the nutrient input.
Date: Oct. 2014 – Ongoing
Funded by: Parks Canada
Co-Funded by: Cooke Aquaculture; Atlantic Canada Fish Farmers Association (ACFFA); Fort Folly First Nation, Fisheries and Oceans Canada (DFO); Province of New Brunswick; University of New Brunswick (UNB)
Collaborators: Cooke Aquaculture; ACFFA; Fort Folly First Nation; DFO; NB; UNB
Contact: info@fundysalmonrecovery.com
Website: www.fundysalmonrecovery.com

In the spring, unfed fry are released for early exposure to the wild environment.
Photo: N. Fearon (Parks Canada)
Integrating genomics within a phenotypic Atlantic Salmon broodstock program
Regulations require that only Saint John River strain Atlantic Salmon are to be raised on the East Coast of Canada, but, European-origin Atlantic Salmon is the basis for the published Atlantic Salmon genome. The ultimate aim of this project is to discover molecular markers pertaining to the specific Atlantic Salmon population and commercial traits of interest.
Since 2010, Huntsman has collected millions of data points on nearly 135,000 individually PIT-tagged Atlantic Salmon that represent 663 families from eight year classes in two generations. Approximately 40,000 fish had finclips archived, following evaluations, to collect data on commercially important traits. These finclips are available for trait marker discovery.
As an initial step, eight finclips were sent to Delta Genomics (University of Alberta affiliated) for genome sequencing; two from each of the four base population year classes used to initiate the Atlantic Salmon broodstock program. The purpose of this initial sequencing was to define options as to whether a commercially available SNP array might have value specific to the Saint John River Atlantic Salmon stock or if developing a stock-specific SNP array represents a better option. This question is valid as the available SNP arrays were originally developed using European stocks of Atlantic Salmon, whereas the local industry in Atlantic Canada must farm the Saint John River stock.
Through the sequencing of individuals from each of the base year classes, the 280K Affymetrix™ SNP chip described 280,255 SNPs. Of these SNPs, 180,316 could be placed on the assembly and had alleles consistent with the European reference genome. Of those SNPs, compared to the eight Saint John River genome sequences, 67,731 SNPs were identified as having the expected alleles and 46,669 showed allelic variation. Therefore, from this commercially available chip, a minimum of approximately 47K SNPs would be polymorphic in our Saint John River Atlantic Salmon population and these polymorphic SNPs are distributed across the published European assembly/reference genome.
Date: Jan. 2017 – Dec. 2018
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-funded by: New Brunswick Innovation Foundation; Northern Harvest Sea Farms
Project Leader: Amber Garber (HMSC)
Project Team: Michelle Miller, Kirill Krivushin, Paul Stothard, Arun Kommadath (Delta Genomics); Chris Bridger (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms)
Contact: amber.garber@huntsmanmarine.ca
Website: Huntsmanmarine.ca
Quantifying direct genetic impacts of escaped farmed salmon on wild salmon populations in Atlantic Canada
Aquaculture escapees represent a demonstrable threat to the persistence and stability of wild salmon populations, with impacts occurring through both genetic and ecological interactions. However, the presence and magnitude of genetic impacts have been difficult to quantify. Our goal is to quantify the extent and magnitude of direct genetic impacts on wild salmon populations due to escaped farmed salmon to inform management decisions and advise on appropriate mitigation strategies.
Specifically, this work addresses three objectives: (1) to quantify the magnitude of low-level chronic escapes through an annual targeted survey (Newfoundland, 2016-2018); (2) to quantify annual variation (2016-2018) in hybridization among wild and farm escaped Atlantic Salmon; and (3) to evaluate survival of hybrids in the wild. By evaluating the occurrence of hybridization among escaped farmed and wild Atlantic Salmon over time, the magnitude of the risk to wild populations of both low-level chronic escapes and large acute events will be quantified. This project directly targets the needs of clients in both aquaculture management and salmon assessment and, for the first time, will allow genetic threats posed by farm escaped salmon on wild populations in Atlantic Canada to be quantified.
Identifying risks and potential mitigation strategies associated with Atlantic Salmon aquaculture escapees is paramount to the successful conservation of wild salmon populations, the stability of recreational and Indigenous fisheries, and the continued growth of the aquaculture industry.
Date: Apr. 2016 – Mar. 2019
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Ian Bradbury (DFO)
Project Team: Steven Duffy, Lorraine Hamilton, Carole Grant, Chris Hendry, Brian Dempson, Ross Jones (DFO);
Collaborators: Ian Fleming (MUN); Jon Carr (ASF); Ross Hinks (NL)
Contact: ian.bradbury@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-NL-02-eng.html
Selecting high-performing pedigreed Atlantic Salmon broodstock
Atlantic Salmon with Northern Harvest Sea Farms is the surrogate species for Huntsman to develop and commercialize effective broodstock development models. In this case, the breeding nucleus is maintained at commercial facilities in PEI, while the random challenge group are held at the Huntsman – both have PIT tagged individuals representing all families within each year class (YC). Huntsman staff travel at strategic times, namely when fish are at 1, 2 and 3 years old, to assess and sort the breeding nucleus fish through ultrasound sexing and the removal of early maturing individuals. Staff also assist during spawning season to produce the future breeding families and to guide spawning for production eggs.
A commercial comparison group with untagged fish from all families of a YC is raised within commercial hatchery and sea cage operations until harvest when Huntsman completes a comprehensive harvest evaluation within the processing plant. During 2017 and 2018, Atlantic Salmon broodstock program activities and milestones included:
- PIT tagging and deformity assessment of 2016/2017 YC;
- Sea lice challenges of 2014/2015 YC;
- Smolt survival challenges of 2015/2016 YC;
- Freshwater critical thermal maximum challenge of 2016 YC to elucidate the effects of climate change;
- Harvest evaluation of 1500 fish from the 2014 YC commercial comparison group, including nearly 30 morphometric and quality traits; and
- Numerous assessments and spawning at the commercial facility in PEI.
Huntsman has extensive infrastructure and expertise to positively effect genetic gains within cultured aquatic species. Developing models that effectively allow these assets to be accessed by numerous companies and sectors across a broad range of species will provide significant impact to regional and national aquaculture sectors. Commercial producers benefit from broodstock management, and producers’ needs to maintain sufficient fish for commercial egg production are also met.
Date: Jan. 2017 – Dec. 2018
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-funded by: New Brunswick Innovation Foundation; Northern Harvest Sea Farms
Project Leader: Amber Garber (HMSC)
Project Team: Susan Hodkinson, Phil Wiper, Brooke Barrett, Chantal Audet, Danny Craig, Howard Streight, Jamie Carpenter, Brian Goggin, Anne McCarthy, Rebecca Eldridge, Elizabeth Dowling, Kelly Greig, Angela Rehhorn, Erica Harvey, Trena Hurley, Ellen Fanning, Dave Goodwin, Farhad Amini, Chris Bridger (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms)
Contact: amber.garber@huntsmanmarine.ca
Website: Huntsmanmarine.ca

Huntsman staff completing commercial harvest evaluation in 2018.
Photo: M. Brown (HMSC)
Genetic and probiotic improvement of immunity in triploid Chinook Salmon
On the west coast of Canada, Atlantic Salmon is still dominant, but Chinook Salmon offers a native species alternative. Escapees mixing with wild stocks can, however, be a potential hazard for this species. One mitigation strategy is to use triploid fish, as they are sterile. This strategy offers the added advantage that triploid fish do not mature sexually, a process that degrades market quality of the fillets. Chinook Salmon maturation is a seasonal process, causing a rush to optimize harvest before the fish mature. Non-maturing triploids will reduce losses due to early maturation of fish and extend the harvest time, providing increased profits. Triploid salmon are more susceptible to disease, however, and currently that prevents the aquaculture industry from obtaining these benefits. The degree of this disease susceptibility differs among families of fish.
This project examines the possibility that triploid fish have compromised immunity due to their genetic background, causing family specific differences in expression of their immune genes, combined with behavioural differences seen in triploids. It will also examine the possibility of ameliorating detrimental effects of triploidy through immunostimulatory probiotic diets.
The results of this research will provide information that might allow the selection of broodstock and diet formulations that will produce triploid fish with minimal extra disease resistance, providing the environmental and economic benefits which triploid fish offer. Additionally, the results of this research will provide information on fish genetics and immunity so novel and unique that it will provide benefits to the aquaculture of all finfish.
Improving the immunity of triploids, through genetics and probiotic diets, and allowing their use in commercial aquaculture will mitigate the environmental impact of escapees and extend the harvest period for greater profits.
Date: Oct. 2018 – Oct. 2021
Funded by: Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Network Program
Co-Funded by: Yellow Island Aquaculture; Taplow Feeds
Project Leader: Brian Dixon (U Waterloo)
Project Team: Dan Heath, Tina Semeniuk (U Windsor); Gregor Reid (U Western); Paul Craig (U Waterloo)
Contact: bdixon@waterloo.ca
Thermal physiology of farmed Atlantic Salmon within a commercial breeding program
Wild fish populations have the ability to geographically shift their range to avoid the long-term effects of climate change, but fish farming lease sites are fixed spatially. A finding of family differences — and therefore heritability — in thermal tolerance will allow this trait to be included in Atlantic Salmon selective breeding indices. This project holds potential to alter how and where salmon farming is undertaken in Canada in future years, especially threatened locations that may otherwise be rendered of no value for this purpose.
This project is assessing the thermal tolerance of Atlantic Salmon parr and post-smolts across more than 186 families, representing two-year classes within a commercial breeding program. While selection programs have revolutionized the industry and allow companies to realize genetic gains for specific traits (e.g., rapid growth, disease resistance, etc.), there is always more work to be done. Global climate change presents a new challenge for the aquaculture industry, and future breeding programs may need to select fish that are better able to withstand and thrive at higher temperatures.
This project will assess the scope for selection of thermal tolerance within the North American-origin Saint John River strain of Atlantic Salmon by examining differences between fish among families following exposure to the same thermal challenge. All challenged fish within a year class will be PIT-tagged and reared communally for the duration of the project. Individuals from each family will be exposed to a critical thermal maximum (CTmax) challenge as parr in freshwater and again as post-smolt in seawater, allowing comparisons to be made both within and among individuals and families across life stages. From there, we will evaluate the effects of this non-lethal temperature challenge on different biological responses (e.g., growth rate) and identify minimally invasive, non-lethal proxies for thermal tolerance for use in commercial breeding programs. One MSc student is being trained with funding through this project.
Date: Sep. 2018 – Aug. 2020
Funded by: ACOA AIF
Co-Funded by: New Brunswick Innovation Foundation, Northern Harvest Sea Farms Ltd., Huntsman Marine Science Centre, University of New Brunswick
Project Leader: Amber Garber (HMSC)
Project Team: Charlotte Bartlett, Tillmann Benfey (UNB); Chris Bridger, Anne McCarthy, Rebecca Eldridge and Elizabeth Dowling (HMSC)
Collaborators: Aaron Craig (Northern Harvest Sea Farms Ltd.)
Contact: cbrownba@unb.ca
Optimizing the performance of triploid fish in aquaculture
Induced triploidy is the only method currently available to produce reproductively sterile populations of fish for commercial aquaculture. Reasons for using such fish include mitigating ecological impacts of escapes, protecting breeding rights, and eliminating precocious sexual maturation. However, their use has been hindered by reduced performance with respect to aerobic stressors such as high temperature, hypoxia, and exhaustive exercise.
Our research and that of others suggests that aerobic scope is reduced in triploids, leaving them with less metabolic reserves to overcome physiological challenges. Using whole-animal respirometry to measure maximum and routine metabolic rates (MMR and RMR) at various acclimation temperatures, we have shown that this is due to high RMR rather than low MMR. We are now probing this in more detail by acclimating fish to different temperatures and then challenging them at high temperatures under progressive hypoxia, and are also investigating the energetic cost of food processing and ammonia excretion, and how these are influenced by temperature and meal size.
Another current line of research is using zebrafish to measure the rate of red blood cell (RBC) synthesis and turnover, as an overabundance of senescent, inefficient RBCs could be driving high RMR in triploids. This could be a direct outcome of their larger genomes, and hence nuclear and cellular volumes, compared to diploids. By defining the conditions under which triploids fail to thrive, and by understanding why they fail, we will be able to optimize rearing practices to facilitate the adoption of triploidy as a standard practice in aquaculture.
There are clear benefits to using triploid (sterile) fish in aquaculture, but these remain largely unrealized because of performance limitations compared to diploids. Our goal is to better understand the underlying reasons for these limitations and thereby improve production protocols for integrating triploids into commercial aquaculture.
Date: Ongoing
Funded by: NSERC (Discovery Grants Program)
Co-Funded by: New Brunswick Innovation Foundation—Research Assistantships Initiative
Project Leader: Tillmann Benfey (UNBF)
Project Team: Chris Small, Krista Latimer, Nicole Daigle, Rebecca Porter (UNBF)
Collaborators: Charles Sacobie (UNBF); Jim Kieffer (UNBSJ); Christine Verhille (MSU)
Contact: benfey@unb.ca
Website: https://benfey.wordpress.com/
Intermittent flow respirometry system for simultaneous measurement of post-feeding metabolic rate in four individual fish.
Video: Nicole Daigle (UNBF)
Mitigating the impact of climate-related challenges on salmon aquaculture (MICCSA)
In this pan-Atlantic applied research project, research tools and products are being developed to: enable the Atlantic Salmon aquaculture industry to prepare for, and monitor, the predicted effects of warming and hypoxic coastal waters; and to develop fish that are better protected from infectious salmon anemia (ISA) and sea lice (Lepeophtheirus salmonis). These goals will be achieved by: 1) defining the sub-lethal and lethal temperatures of current Atlantic Salmon stocks (i.e., fish of Saint John River origin); 2) studying how fish with varying genetic backgrounds perform under conditions of hypoxia and elevated temperature; 3) developing molecular markers (single nucleotide polymorphisms, SNPs) for selecting broodstock with high temperature tolerance, robust immune responses and improved disease and stress resistance; and 4) developing genomic and antibody-based diagnostic assays for assessing fish health and producing improved, more effective, vaccines.
This research program has already: validated the use of data loggers that simultaneously record heart rate, activity/swimming speed and body temperature that can be used to monitor free-swimming Atlantic Salmon in sea cages; provided significant data on how high temperatures (20–23˚C) alone, or in combination with moderate hypoxia, impact production characteristics, stress physiology, and the salmon’s immune response to viral and bacterial antigens (i.e., vaccination); identified several key immune- and stress-related genes (biomarkers) that are responsive to temperature / hypoxic challenges; and produced antibodies and ELISAs (Enzyme-Linked Immunosorbent Assays) to several biomarkers so that their protein levels can be quantified and monitored.
The results of this project may lead to an improved capacity to monitor fish welfare and health, and may contribute to improved broodstock that can tolerate climate-related challenges and are more resistant to important diseases.
Date: Sep. 2016 – Sep. 2022
Funded by: Atlantic Canada Opportunities Agency – Atlantic Innovation Fund (ACOA – AIF)
Co-Funded by: Innovate NL; Innovate PEI; Memorial University; University of Waterloo; Huntsman Marine Science Center; Center for Aquaculture Technologies Canada; Somru BioScience
Project Leader: Kurt Gamperl (MUN); Mark Fast (UPEI)
Project Team: Brian Dixon (U Waterloo); Matt Rise (MUN); Roy Danzmann, Fabio Zanuzzo, Anne Beemelmanns, Aaron Frenette, Olufemi Ajiboye, Zoe Zarini, George Heath, Rebeccah Sandrelli, Ellen Peroni, Tanya Rodríguez-Ramos, Shona Whyte, Sara Purcell (U Guelph); Danny Boyce (JBARB)
Collaborators: Amber Garber, Chris Bridger (HMSC); Tiago Hori (CATC); Jessi Rix (Somru BioScience)
Contact: kgamperl@mun.ca

Laboratory for Atlantic Salmon and Climate Change Research (Ocean Sciences Center, MUN).
Photo: Kurt Gamperl (MUN)
Enhancing production in Coho: Culture, community, catch (EPIC4)
Coho Salmon, one of the most highly valued species in British Columbia (BC), began suffering declines in 1989 due to lower returns and high harvest rates, to the point where the commercial fishery for Coho Salmon was essentially closed in 1997. Reopening the Coho Salmon fishery using recovered and enhanced populations would bring economic and social benefits to BC.
The EPIC4 project aims to develop and use new genomics tools to address challenges facing safe, secure and sustainable production of Coho Salmon. The interdisciplinary team has sequenced the Coho Salmon genome and the first results have resolved clear patterns of regional population structuring, both within BC and across the entire distribution range. Genotyping of the hatchery broodstocks sampled in 2014 and 2015 showed strong regional population structuring and allowed high levels of accuracy in assigning salmon to specific hatcheries or geographic regions. In addition, first results on heritability and genetic correlation shed light on the genetic basis of flesh colour and the response of this trait to artificial selection for harvest weight over the time course of eight generations.
The team is also working with stakeholders, including First Nations, regarding the implementation of EPIC4 scientific knowledge about Coho Salmon to help revive and sustain the wild Coho fisheries. The work on this project could lead to more economically viable Coho Salmon fisheries serving both domestic and export markets. Our results should also be transferable to other species of Pacific salmon, as well as salmonids from other regions of Canada.
Date: Oct. 2015 – Sep. 2020
Funded by: Genome Canada; Genome British Columbia
Co-funded by: Genome Quebec; Fisheries and Oceans Canada (DFO); University of Victoria; Benchmark Genetics Chile; Resources Aquatiques Quebec (RAQ); Institut de Biologie Intégrative et des Systèmes (IBIS); Thermofisher
Project Leader: Ben Koop (UVic); William S. Davidson (SFU); Louis Bernatchez (U Laval)
Project Team: Roberto Neira, Jose Yanez (U Chile); Terry Beacham (DFO); Grant Murray (VIU, Duke U); Kerry Naish (U Washington); Rashid Sumaila, Ralph Matthews (UBC); Steven Jones (SFU)
Collaborators: Robert Devlin, Ruth Withler, David Willis (DFO); Brian Riddel (Pacific Salmon Foundation); Jean Paul Lhorente (Aquainnovo)
Contact: ksivak@sfu.ca
Website: http://www.epic-4.org

Spawning day at Inch Creek Hatchery. The staff are measuring and recording the phenotypic data from 2017 female broods.
Photo: Michelle TT Crown (SFU)
Sea lice
Susceptibility of farmed and two origins of wild Atlantic Salmon (Salmo salar) to experimental infestations with sea lice (Lepeophtheirus salmonis)
Sea lice (Lepeophtheirus salmonis) are common pests on farmed Atlantic Salmon and can have large economic consequences for the salmon industry. Since sea lice may use light as a cue to make contact with the host fish, the project assessed whether the light regimen in a tank (full time low light vs alternance between light and dark) affected the settlement patterns of sea lice on salmon post-smolts, taking into account fish size and fin erosion. Results demonstrated that the light regimen did not influence sea lice infestation levels, but did impact distribution of sea lice on the host body. Additionally, fin erosion did not have a significant effect on total sea lice counts, but affected proportional distribution of sea lice. Increased fin erosion lowered the number of sea lice observed on paired fins and increased sea lice on the other anterior parts of the fish body.
This project also utilized controlled sea lice laboratory infestations to evaluate the susceptibility of three populations of Atlantic Salmon to sea lice. Wild salmon from two origins (Garnish River and Conne River) of Newfoundland’s South coast and farmed salmon were exposed to sea lice for a period of 26 days. It was observed that Conne River fish had significantly higher sea lice densities than farmed salmon and wild salmon from Garnish River, suggesting that genetic differences influence susceptibility. Differences in sea lice locations were identified among the salmon populations, particularly: 1) more sea lice on gills of farmed fish compared to Conne River salmon, likely due to differences in gill vascularization and localized immune responses; 2) more sea lice on paired fins of wild populations (significant only in Garnish fish) compared to farmed fish, linked to a greater fin surface area in wild salmon due to higher erosion in farmed smolts. In addition, wild and farmed infested salmon presented different dose-responsive up-regulation of skin key immune-relevant genes. This result highlights a discrepancy in both innate and adaptive (local and systemic) immune system function between wild and farmed salmon.
Date: Apr. 2015 – Mar. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Cold Ocean Salmon Inc.
Project Leader: Dounia Hamoutene (DFO)
Project Team: Lynn Lush, Kimberley Burt, Daria Gallardi, Harry Murray (DFO)
Collaborators: Julia Bungay (Cold Ocean Salmon Inc.)
Contact: Dounia.Hamoutene@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-N-01-eng.html
The lethal and sublethal effects of anti-sea lice therapeutants on marine benthic and pelagic invertebrates
The environmental consequences of aquaculture chemotherapeutants used as a treatment for sea lice were assessed by generating data on the effects of these chemicals on pelagic and benthic non-target marine organisms under realistic exposure concentrations and durations. Currently, there is specific concern regarding bath chemotherapeutant toxicity to planktonic organisms or to those that inhabit the water column for specific life stages (e.g., gametes or larvae). Both lethal and sublethal effects associated with short-term pulse exposures under environmentally realistic concentrations are known in only a few specific planktonic species (or life stages) found on the East coast of Canada and in the marine waters of Europe. Here, a series of studies were conducted that expand currently funded research using targeted representative Pacific marine species (bivalves and echinoderms).
In current experiments, reproduction and development were examined in both adult (fertilization/gametes) and larval stages (development) of purple sea urchin (Stronglylocentotus purpuratus) and blue mussel (Mytilus edulis) that would be found in the water column to determine the lethal and sublethal effects of both Salmosan® and Paramove® 50. Benthic and epibenthic organisms are at risk of exposure to both ivermectin and SLICE® that partition to sediments. Subchronic sediment exposures (>28 days) were incorporated into experiments aimed at determining the sublethal toxicity of these chemicals for these potentially susceptible groups of organisms. Effects on organism avoidance and locomotory behaviour, oxygen consumption, as well as effects on growth were measured.
This research provides targeted information on the lethal and sublethal toxicity of these compounds, focusing on exposure pathways and durations that are most environmentally relevant. These data are required to ensure the proper and safe use of these chemicals through science-based regulations.
Date: Sep. 2017 – Mar. 2018
Funded by: DFO–National Contaminants Advisory Group (DFO–NCAG)
Co-Funded by: Simon Fraser University (SFU)
Project Leader: Chris Kennedy (SFU)
Project Team: Lindsay Woof, Samantha Lunquist, Stephanie Cooper, Munraj Bajwa (SFU)
Collaborators: Nautilus Environmental
Contact: ckennedy@sfu.ca

Behavioural assay tanks for polychaetes (Attila virens) exposed to chemotherapeutants.
Photo: Lindsay Woof (SFU)
Defining the risk of sea lice infections through the development of an understanding of the early life history population dynamics of sea lice on Atlantic Salmon aquaculture sites in the Bay of Fundy
This is an important time for the salmon farming industry on the East coast as there are signs that resistance is developing in sea lice to the current suite of therapeutant sea lice treatments resulting in more intense infection pressures on the salmon. This is not only hindering current farming operations, but also future development, thereby creating a demand for more effective alternative approaches to parasite control.
This project will seek to better understand the distribution and dynamics of sea lice larvae originating from salmon farms to help identify specific options for treatment or farm management techniques to target sea lice larvae and reduce the overall frequency and/or severity of sea lice infestations on salmon farms in the Bay of Fundy. As sea lice are currently one of the major issues affecting the success of salmon farming, and very little information is known on the early life history of this species, this information will be crucial to successfully manage this issue.
This project is designed to combine both lab and field components to understand the mechanisms behind the infection dynamics of larvae finding and attaching to their host fish. It is providing some of the first views of larval sea lice densities in proximity to salmon farms in the Bay of Fundy and how they vary over time and space.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cooke Aquaculture Inc.
Project Leader: Shawn Robinson (DFO)
Project Team: Emily Nelson (DF0)
Collaborators: Keng Pee Ang (Cooke Aquaculture Inc.)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-M-01-eng.html
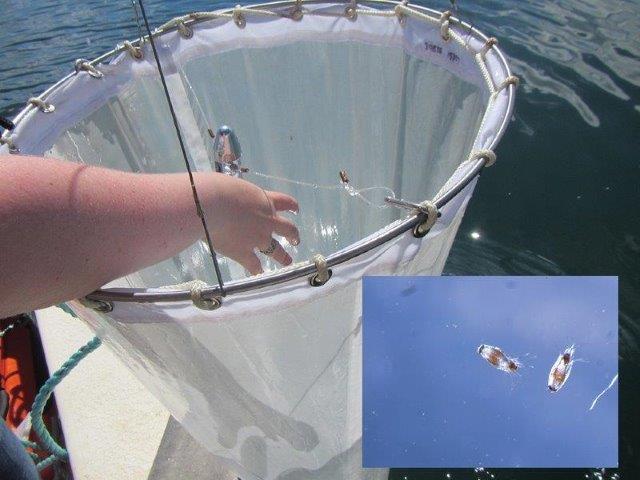
Plankton net used to sample larval sea lice in and around salmon cages with an inset showing a larval sea louse.
Photo: S. Robinson (DFO)
Effects of organophosphate aquaculture pesticide Azamethiphos on American Lobster (Homarus americanus) larvae (stage I, II, III)
Both the American Lobster fishery and Atlantic Salmon aquaculture are important to the economy of Atlantic Canada. Research is needed to better understand any interactions and their possible effects given that these industries often operate in close proximity.
Laboratory exposures have revealed various pesticide effects on lobster larval survival, behaviour, development and immunity. This study explored the effects of Salmosan® WP50 – active ingredient azamethiphos – on the survival and development of planktonic early life stages of American Lobsters, Homarus americanus. Salmosan® is used to treat Atlantic Salmon infestations with parasitic sea lice.
At each planktonic stage (Stages I-III), individual lobsters were exposed for three hours to water-borne Salmosan® at six treatment concentrations ranging from 0.21 to 42 μg L-1 azamethiphos, with 30 individuals per treatment. Treatment-related effects were observed, with higher concentrations causing increased mortality and decreased moulting success. Significant effects on immobilization were observed after three hours of exposure to concentrations as low as 2.8 μg L-1 azamethiphos. Acethylcolinesterase (AChE) response proved consistent across stages in terms of acting as a biomarker for exposure. Decreasing AChE activity was significantly correlated with increased immobilization and mortality, and activities below 1.1, 1.2 and 22.9 nmol/min mg for Stage I, II and III, respectively, may indicate significant immobilization and predict future mortality of those stages. The temporal and geographical overlap of larval lobster life stages and the use of Salmosan® is important to consider when assessing potential risk.
This research provides information on sensitive life stages of a non-target organism under environmentally realistic conditions, thereby improving risk assessments, and providing valuable input to support integrated pest management strategies on aquaculture sites.
Date: Jun. 2017 – Oct. 2017
Funded by: DFO-National Contaminants Advisory Group (DFO-NCAG)
Project Leader: Dounia Daoud (Homarus Inc.)
Project Team: Benjamin de Jourdan, Anne McCarthy, Rebecca Eldridge, Ellen Fanning, Elizabeth Dowling, Erica Harvey, Aldea Poirier, Emma Bowland, David Goodwin (HMSC)
Collaborators: Marc Surette (U Moncton)
Contact: dounia@mfu-upm.com
Website: http://www.homarus.org/

Eggs develop on the abdomen of a berried female American lobster (top left), then hatch to become planktonic Stage I larvae (top right). These larvae molt to become Stage II larvae (middle right), then molt to become Stage III larvae (bottom right). All of these planktonic larval stages were exposed to complete the project.
Source: Huntsman Marine Science Centre
Spatial and temporal patterns of sea lice infestations on wild and farm-raised salmon on the British Columbia coast
In 2016, UPEI and the BCSFA initiated the Wild Salmon Sea Lice Integration Project (WSSLIP), aggregating data from around seventy different sources, which has led to the largest collection of sea lice records relating to wild salmon to date anywhere in the world. By the end of 2017, data had been collected from almost one million wild Pacific salmon at over three hundred sampling locations along the BC coast over the previous sixteen years. From these, almost 250,000 fish captured at around 12,000 separate events had been assessed for sea lice infestation.
A project funded through the BCSFA’s Marine Environmental Research Program (MERP) enabled the use of this large database on sea lice infestation in wild Pacific salmon for the study of spatial and temporal patterns of sea lice infestation in both wild and farmed salmon. The initial work focused on collecting comparative data from BC salmon farms over a similar period. In addition to sea lice levels on around 220,000 farmed fish from over a hundred sites, data on around 550 cycles of Atlantic Salmon production and 675 treatment events have been integrated into the data set. Work is now underway to complete a peer-reviewed manuscript, which will summarize and describe trends and associations within these data.
This exploration of spatial and temporal patterns of sea lice infestation in the Pacific will eventually allow the development and testing of predictive models of sea lice infestation that could help mitigate their impacts.
Date: Apr. 2016 – Mar. 2018
Funded by: British Columbia Salmon Farmers Association–Marine Environmental Research Program (BCSFA–MERP)
Project Leader: Crawford Revie, Thitiwan Patanasatienkul (UPEI)
Collaborators: Joanne Liutkus (BCSFA)
Contact: joanne@bcsalmonfarmers.ca
Website: BCsalmonfarmers.ca
Characterizing function of enzymes in the chitin synthesis pathway of Lepeophtheirus salmonis
As an arthropod, the salmon louse (Lepeophtheirus salmonis) relies on the production of chitin. Drugs targeting chitin synthesis have been largely successful against terrestrial parasites, where the pathway has been well characterized. However, a comparable approach against L. salmonis has been less successful, likely due to a poor understanding of the chitin synthesis pathway (CSP). RNA interference (RNAi) is a powerful method for evaluation of protein function in nonmodel organisms and has been successfully used to deduce function of several genes in L. salmonis. Thus, the purpose of this work was to use RNAi to characterize the function of putative genes in the CSP of L. salmonis.
Using this technique, we demonstrate complete abrogation of infectivity in larval L. salmonis after knockdown of key CSP enzymes, glucosamine: fructose-6-phosphate aminotransferase (GFAT), or chitin synthase (CHS). Both groups of treated sea lice were observed to molt to the infective copepodite stage but were unable to maintain position in the water column or attach to a host fish. Sea lice with the CHS1+2 knockdown did not present any obvious phenotypic variation; however, in contrast, the GFAT knockdown was accompanied by a severe phenotypic aberration whereby all appendages were thickened (as can be seen in the picture).
This work demonstrates the importance of the chitin synthesis enzymes GFAT and CHS for successful infectivity of L. salmonis. Furthermore, this work highlights the applicability of RNAi as a tool to explore novel targets for sea lice control.
Date: Jan. 2017 – Jan. 2019
Funded by: Elanco Animal Health
Co-Funded by: Norwegian Research Council; Natural Sciences and Engineering Council of Canada (NSERC)
Project Leader: Mark Fast (AVC)
Project Team: Laura Braden, Okechukwu Igboeli, Michael Dundrop, Lars Hamre, Sussie Dalvin, Christiane Eichner, Frank Nilsen, Jordan Poley (UPEI)
Collaborators: Sea Lice Research Centre (U Bergen)
Contact: lbraden@upei.ca

Copepodite L. salmonis after knockdown of GFAT using RNAi showing extreme phenotypic anomaly.
Photo: Laura Braden (AVC/AquaBounty)
Larvae with GFAT knocked down are unable to swim as shown here. Video: Laura Braden (AVC/AquaBounty)
To control for the effect of knockdown, a group of larvae were treated with dsCYP, a cod gene. As shown in the video, these animals are behaving typically, showing phototactic behaviour, and rapid swimming. Video: Laura Braden (AVC/AquaBounty)
Effects of new and existing anti-sea lice in-feed treatment options on voluntary feeding juvenile American Lobsters (Homarus americanus)
The American Lobster fishery often co-exists with Atlantic Salmon aquaculture. Regulators must therefore understand possible effects of anti-sea lice treatment drugs on the lobster, as a non-target species that might consume uneaten dosed feed or faeces that pass through treated fish cages.
Juvenile lobsters are too small to allow forced feeding that ensures delivery of a known dose. Instead, toxicology relies on offering dosed fish feed pellets to lobsters for voluntary feeding. This approach allows estimation of lethal concentration values associated with novel in-feed anti-sea lice drugs in post-settled lobsters. The study methods of this work included:
- Establishing replicate study tanks that each contain five cells to hold individual juvenile lobsters. Each tank holding five individuals is an experimental unit, with replicates randomly assigned within blocks throughout the controlled access study wet laboratory.
- Maintaining lobsters through acclimating, fasting, dosing and post-dosing periods. Dosing matches the anticipated drug treatment period to Atlantic Salmon (e.g., 7-day dosing period). The post-dosing period is at least 28-days to provide time for delayed effects.
- Offering a set number of dosed feed pellets daily to each juvenile lobster
- Siphoning uneaten whole or portions of feed pellets the following morning before the next pellet offering. Drying collected feed pellets to weigh the uneaten pellets and therefore determine missing, possibly consumed, feed.
- Observing treated juvenile lobsters daily within their individual cells during the dosing and post-dosing periods and documenting observations using an established scoring system and series of standardized acronyms to describe behaviour.
The described voluntary consumption exposure model provides essential toxicology data for early life stages of this commercially important, non-target species associated with new and existing anti-sea lice in-feed treatment options. Several studies using this specific design have been completed involving candidate anti-sea lice drugs that have different modes of action.
Date: Sep. 2015 – Dec. 2017
Funded by: Huntsman Marine Science Centre (HMSC)
Project Leader: Les Burridge (HMSC)
Project Team: Anne McCarthy, Esther Keddie, Trena Hurley, Rebecca Eldridge, Louise Warner, David Goodwin, Benjamin de Jourdan, Chris Bridger (HMSC)
Contact: chris.bridger@huntsmanmarine.ca
Website: Huntsmanmarine.ca

Lobster holding tank set-up with four rows of fifteen tanks. Each tank provides five cells to individually house juvenile lobsters to facilitate individual observations and offer protection (n = 300 lobsters total in the photo).
Photo: Huntsman Marine Science Centre
Development of diets and feeding strategies for the implementation of sea lice cleaner fish in salmon farms in BC
This project is an integral component of a larger project to establish a commercial perch sea lice cleaner fish industry to support salmon aquaculture in BC. Research to date has provided clear evidence that Kelp and Pile Perch are efficient at cleaning sea lice off infested salmon. In order for this research to advance to the next stage, it is vital to determine the optimal diets to feed the perch for sustained growth, health and welfare both prior to and after deployment in the farms. Healthy and robust cleaner fish have been shown to be the most effective at cleaning sea lice, which makes this research very important to this new industry.
The overall objective of this research project is to develop diets and feeding strategies for Kelp and Pile Perch, in support of their use as sea lice cleaner fish in Atlantic Salmon farms in BC. The information gathered from this research will provide valuable information required to establish a hatchery to produce the perch for deployment in the salmon net pens. This research will help develop diets for the perch at all life history stages, and the most appropriate feeding systems for use in the net pens.
Date: Apr. 2017 – Mar. 2019
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: BC Salmon Farmers Association (BCSFA)
Project Leader: Ian Forster (DFO)
Project Team: Shannon Balfry (CAHS); Mirko Diaz, Katarina Doughty (DFO)
Collaborators: BCSFA
Contact: Ian.Forster@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-P-03-eng.html
Effects of new and existing anti-sea lice in-feed treatment options on force fed adult American Lobsters (Homarus americanus)
Candidate anti-sea lice treatment drugs must be extensively studied before national regulators will allow use within the local seawater environment. Characterization of efficacy, target animal safety, pharmacokinetics, etc., are necessary. Also required are studies focused on environmental effects, such as those on local non-target species (e.g., the American Lobster in Atlantic Canada).
Staff at Huntsman refined earlier methods to acquire lethal dose values associated with novel in-feed sea lice drugs in adult American Lobsters. In a series of experiments, we used these methods to assess the following dose responses:
- Providing a known range of doses delivered to individual test lobsters in spiked fish food slurry via oral gavage. Lobsters are known to be messy feeders and therefore voluntary consumption on dosed pellets is not expected to provide accurate estimation of actual received dosage. This is an important consideration in order to determine dose response relationships and establish critical tissue body burden.
- Holding dosed lobsters within individual aquaria for a set period of time to ensure regurgitation of spiked slurry does not occur.
- Observing dosed lobsters within individual grid cells for several days with daily observations using an established scoring system and series of standardized acronyms to describe behaviour. This phase is most important for neurotoxic molecules.
- Maintaining dosed lobsters that survived beyond the initial observation period for an extended timescale post-dosing (typically 12–24 months) to monitor for delayed effects.
The described exposure model allows for more accurate risk assessments associated with new and existing anti-sea lice in-feed treatment options. Several non-target adult American Lobster studies using this specific design have been completed on behalf of pharmaceutical companies to estimate lethal dose values following extended post-dosing holding periods for candidate anti-sea lice drugs having different modes of action.
Date: Jan. 2015 – Sep. 2017
Funded by: Huntsman Marine Science Centre (HMSC)
Project Leader: Les Burridge (HMSC)
Project Team: Anne McCarthy, Esther Keddie, Trena Hurley, Sarah Ogilvie, Erin Carpenter, Rebecca Eldridge, David Goodwin, Benjamin de Jourdan, Chris Bridger (HMSC)
Contact: chris.bridger@huntsmanmarine.ca
Website: Huntsmanmarine.ca

Oral gavage of adult American Lobster with a spiked fish food slurry
Photo: A. McCarthy
Pile Perch (Rhacochilus vacca) as a cleaner fish for sea lice on farmed salmon: identification of pathogens of concern with respect to salmon and perch health
The presence of sea lice is a significant economic burden to commercial salmon aquaculture. Since 2001, dietary emamectin benzoate (EMB) had been the treatment of choice for sea lice on farmed Atlantic Salmon because of its high efficacy and ease of application. However, the development of EMB resistance among sea lice populations has reduced its usefulness as a treatment in Norway, Chile and Eastern Canada. In British Columbia, reports of variable EMB treatment efficacy suggests that resistance to EMB may be developing, stressing the need to find alternative tools for controlling sea lice.
In Norway, cleaner fish are commonly used to control sea lice infestations and reduce the reliance on chemical treatments. In BC, the potential for using native Pile Perch as cleaner fish to control sea lice has been demonstrated in the laboratory. However, prior to commercial development and deployment of Pile Perch as a cleaner fish, it is necessary to understand the potential fish health risks, if any, posed by their use. It is also important to identify any infectious agents that may directly affect the Pile Perch. This project will examine pathogens of concern to salmon and perch health, and seek to identify if Pile Perch could act as an alternative or reservoir host of salmon pathogens.
Date: Apr. 2018 – Jun. 2019
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: BC Salmon Farmers Association (BCSFA)
Project Leader: Stewart Johnson (DFO)
Project Team: Diane Morrison (Marine Harvest Canada); Ahmed Siah (CAHS)
Collaborators: Joanne Liutkus (BCSFA)
Contact: stewart.johnson@dfo-mpo.gc.ca
The environmental effects of anti-sea lice pesticides on marine benthic species
Strategic chemotherapeutic treatments for sea lice control are essential to finfish production in intensive aquaculture. Health Canada assesses and approves chemotherapeutants for use in the salmon aquaculture industry; these are considered either a drug (if administered in feed) or a pesticide (if administered in a water bath). Predictions of the persistence and toxicity of these chemicals to non-target organisms have been undertaken in Canada and globally where salmon farming occurs, but it is recognized that data gaps exist for various species, including Pacific benthic species. Regardless of partitioning behaviour, benthic and epibenthic organisms are at risk of exposure to some or all of these chemicals via overlying water, pore water, or sediments, depending on the fate of the compounds and environmental conditions. Because of this potential for exposure, the chemotherapeutant toxicity to benthic fauna near aquaculture operations should be better understood.
This project aims to incorporate both water and sediment exposures as well as acute and chronic exposure time frames into experiments aimed at determining the lethal and sublethal toxicity of these chemicals in potentially susceptible organisms. Specifically, this research is focused on environmentally realistic exposures of chemotherapeutant formulations (short term water exposures for Salmosan® and Paramove® 50 and long term sediment exposures for ivermectin and SLICE® and a combination of both) on benthic animals (annelids, crustaceans, and fish). Lethal studies were performed on polychaetes, amphipods, spot prawn and starry flounder. Sublethal endpoints examined in spot prawn and starry flounder in these experiments include: locomotion and behaviour, stress and oxygen consumption, molting (spot prawn), and growth. The data obtained from this research will ensure the proper and safe use, and the appropriate regulation of these aquaculture chemicals in Canada.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO – National Contaminants Advisory Group (DFO–NCAG)
Co-Funded by: Simon Fraser University (SFU)
Project Leader: Chris Kennedy (SFU)
Project Team: Stephen Barrett, Kate Mill, Daniel King, Tooba Khan, Michael Dong, Manpreet Jhutty, Annie Cai, Michelle Young, Karan Parekh (SFU)
Collaborators: Nautilus Environmental
Contact: ckennedy@sfu.ca

Exposure tanks for amphipods (Eohaustarius estuarius) used for short term exposures to water contaminated with Salmosan® and Paramove® 50.
Photo: Lindsay Woof (SFU)
Refining the use of warm water showers to remove sea lice from Atlantic Salmon and understanding the fish health implications of the technique
Considerable effort is expended (via chemotherapeutants and animal husbandry practices) to manage parasitic infections by the sea louse, Lepeophtheirus salmonis, a globally acknowledged challenge for salmon farmers. The parasite is gaining resistance to treatments and new approaches are urgently needed.
A new technology was introduced to salmon farming in the Bay of Fundy in which salmon are exposed to a warm water shower. This technology was found to be very effective at removing mobile stages of sea lice and was able to retain virtually all of the removed sea lice, preventing them from being reintroduced into the water column near the salmon cages and re-infecting the fish.
This project confirmed that a warm seawater spray can effectively and regularly remove up to 95% of the mobile stages of the salmon sea louse. A new prototype system, using a stainless-steel trough configuration, worked very well and allowed for increased throughput of fish through the shower. The new designs, in addition to being more cost-effective, are much better at handling any mucus removed from the fish and the resulting foam production in the filter systems. The optimal temperature of the shower system is 32 to 34°C, with an exposure time of 25 to 30 seconds. Fish remain healthy during the treatment; any immunological impairment was resolved within 48 hours.
Overall, this project confirmed that the warm water shower technology can effectively control parasitic sea lice infections in salmon aquaculture. This new, environmentally benign treatment option for industry will increase the sustainability of the sector.
Date: Jul. 2016 – Jun. 2018
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd. (KCS)
Project Leader: Shawn Robinson (DFO)
Project Team: Steve Neil, Craig G. Smith (DFO); Joel Halse (KCS)
Collaborators: Keng Pee Ang (KCS); Duane Barker (HMSC)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-M-01-eng.html

Aerial view of the Cooke R warm water shower at a salmon farm in the Bay of Fundy.
Photo: J. Day (DFO)
The environmental effects of anti-sea lice pesticides on marine zooplankton
The quality of near-shore coastal waters and estuaries is of great concern to Canadians, particularly as these ecosystems become increasingly threatened by pollution. In addressing this priority, an improved understanding of chemical impacts on near-shore ecosystems is essential to the responsible stewardship of Canada’s coastal areas. In recent years, the salmon aquaculture industry has become a major contributor to the Canadian economy and, as is the case globally, this industry relies on the strategic use of chemotherapeutic treatments (drugs and pesticides) to control parasitic sea lice, which can result in the release of these products within local net pen areas. Typically, sea lice are managed through harvesting, topical pesticides or in-feed medications. Pesticides, approved for use by Health Canada to treat sea lice, come with label directions to protect non-target species. However, there is interest in a better understanding of the conditions under which the use of these products could pose a risk to sensitive non-target organisms.
Zooplankton play a key role in marine food web dynamics, biogeochemical cycling, and fish recruitment. However, despite their importance in marine environments, our knowledge of the interactions between zooplankton and aquaculture chemotherapeutants is limited. Research is currently underway involving a series of studies using natural zooplankton assemblages, and targeted representative Pacific zooplankton species (crustacean larvae, herring larvae, and copepods) to determine the acute lethality of two chemical therapeutants for sea lice treatments, namely Salmosan® and Paramove®50 formulations, under realistic exposure concentrations and durations, as well as to examine the sublethal effects of these exposures on the growth, development, and reproduction of a marine copepod species.
This research specifically addresses information gaps that need to be filled in order for proper assessments to be made of the environmental (non-target organism) consequences of Salmosan® and Paramove®50 formulations’ use in Canada.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Simon Fraser University (SFU)
Project Leader: Chris Kennedy (SFU)
Project Team: Jenna Keen, Ashish Patankar, Vivian Tsui (SFU)
Contact: ckennedy@sfu.ca
Website: The environmental effects of anti-sea lice pesticides on marine zooplankton

MET graduate student, Jenna Keen, sampling wild zooplankton for exposure to Salmosan® and Paramove® 50.
Photo: Jenna Keen (SFU)
Assessing the potential for development of thermal resistance of sea lice to warm water showers
Outbreaks of sea lice are a globally acknowledged challenge for salmon farming operations; they are a risk to wild salmon populations and a considerable amount of resources are being expended by industry and governments from many countries in order to manage these pests. Chemotherapeutants and animal husbandry practices have been traditionally used to keep sea lice under control, but the parasite is becoming resistant to many of the chemicals that are being used. There are strong incentives to look at other non-chemical approaches to dealing with sea lice.
One of the more promising techniques being developed to remove sea lice from farmed salmon and to control the attached stages is the use of warm water showers. Previous ACRDP project results have demonstrated that the use of a warm water shower can effectively remove up to 95% of the mobile stages of sea lice from the fish in a thirty second treatment, with very low mortality rates in the fish. A recent study has suggested the possibility that resistance of sea lice to the warm water or freshwater treatments may develop over time, in a manner similar to resistance to chemotherapeutants.
The overall goal of this project is to test the concept that sea lice may develop resistance over multiple generations to the temperatures currently being used in the warm water shower to such an extent that the declining efficiency of the warm water shower eventually renders it useless. If this resistance occurs, understanding the rate of this potential change in resistance to warm water by the sea lice will enable better planning for the lifespan of this technology and the timescale for alternatives to be developed.
Date: May 2018 – Mar. 2021
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd.
Project Leader: Shawn Robinson (DFO)
Project Team: Hannah Bradford (DFO); Joel Halse (Cooke Aquaculture Inc.)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-m-02-eng.html

Female salmon sea louse (Lepeophtheirus salmonis) with egg strings containing 300-400 eggs.
Photo: P. Robertson (DFO)
Underwater drones lead the way to more opportunities in cleaner fish behaviour research within Atlantic Salmon sea cages
Two cleaner fish species, the Lumpfish (Cyclopterus lumpus) and the Cunner (Tautogolabrus adspersus), have recently begun to be used to remove sea lice from Atlantic Salmon in commercial sea cages in Atlantic Canada. However, key aspects of cleaner fish ecology and behaviour remain poorly known including their diet within the diverse cage environment, how they are spatially distributed, and how often they interact with their client salmon.
Our research incorporated the use of an inexpensive underwater drone, the Gladius Advanced Pro, to obtain a good spatial understanding of cleaner fish distribution throughout a commercial sea cage. The mobility of this drone allowed us to inspect the usefulness of different artificial hide designs, locate aggregations of cleaner fish, provide insights into their feeding behaviour, and track daily cleaner fish activities throughout the cages. Videos from the drone allowed for quantification of a variety of cleaner fish behaviours that can be used to estimate the cleaner fish population’s overall cleaning efficacy of sea lice within a sea cage. The video observations will be compared with morphological and molecular metabarcoding of stomach contents of different sizes of cleaner fish from the cages and from the wild. Sea lice prevalence in stomachs from different families will be compared and used to select parents for cleaner fish breeding programs.
Our results will provide direction for future development of an optimal cleaner-client environment within Atlantic Salmon sea cages. We are working towards determining the spatial and temporal mapping of cleaner fish distributions throughout the cages, recording deep-water intraspecific and interspecific interactions, and striving to develop cleaning stations that promote interactions between cleaner fishes and their Atlantic Salmon client.
Date: Oct. 2017 – Sep. 2020
Funded by: Natural Sciences and Engineering Research Council of Canada (NSERC) — Strategic Partnership Grant for Projects
Co-Funded by: Cooke Aquaculture Inc.
Project Leader: Elizabeth Boulding (U Guelph)
Project Team: Camden Moir, Jessica Roy, Andjin Siegenthaler, Larry Schaeffer (U Guelph)
Collaborators: Keng Pee Ang, Jake Elliott, Sheldon George, Marine Herlin, Geoffrey McBriarty, Frank Powell (Cooke Aquaculture Inc.); Thor Magne Jonassen (Akvaplan-niva)
Contact: Boulding@uoguelph.ca
Website: https://www.uoguelph.ca/ib/boulding
Video of Lumpfish in the sea cages.
Video: Jessica Roy (U Guelph)

The Gladius Advanced Pro drone in action.
Photo: Camden Moir (U Guelph)

Jessica Roy and Camden Moir operating the drone.
Photo: Jessica Roy (U Guelph)
Genomic assessment of potential genetic interactions of the use of European Lumpfish as a cleaner fish in Atlantic Salmon aquaculture in Newfoundland
The effective management of sea lice continues to be a priority for the Atlantic Salmon aquaculture industry and as such the use of cleaner fish, such as Common Lumpfish (Cyclopterus lumpus), has become an increasingly common alternative to chemical pesticides. Recent research has indicated that both escaped farmed salmon and other translocated cleaner fish species can hybridize with wild populations and pose a threat to the genetic integrity of wild populations. Given declines in wild Common Lumpfish over the last twenty years in Atlantic Canada, the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) has recommended a “threatened” listing for Common Lumpfish. As such, there remains considerable uncertainty regarding the potential impact of the use of Common Lumpfish in salmon aquaculture on local wild populations.
This project will characterize the population structure of wild and cultured lumpfish populations in Atlantic Canada and Europe. Genome-wide screening and extensive sampling will be used to: (1) identify stock structure among wild populations of Common Lumpfish in Atlantic Canada; (2) directly compare wild Common Lumpfish to both European and domestic aquaculture strains; and (3) develop and test targeted SNP panels for the identification of wild-cultured hybrids. This project will both directly inform aquaculture management and Common Lumpfish conservation regarding the risks of importation of European Lumpfish, and provide the tools to quantify potential genetic interactions resulting from their use in salmon aquaculture.
Date: Apr. 2018 – Mar. 2021
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Project Leader: Ian Bradbury (DFO)
Project Team: Amber Messmer, Steven Duffy, Johanne Gauthier, Mark Simpson, Chris Hendry, Helen Griffiths (DFO)
Collaborators: Matthew Kent (NMBU); Danny Boyce (MUN); Paul Bentzen (Dalhousie U)
Contact: ian.bradbury@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2018-nl-04-eng.html

Electrofishing for juvenile Atlantic Salmon.
Photo: KÖBB Media / DFO

Electrofishing for juvenile Atlantic Salmon.
Photo: KÖBB Media / DFO
Spatial heterogeneity in sea lice infestations on farmed salmon in British Columbia
In British Columbia, emamectin benzoate (EMB) has been an effective treatment for salmon lice since 2000. Since 2004, sea lice management has been linked to wild salmon conservation with coast-wide regulations that ensure motile sea lice abundance remain below 3 per salmon between March and July, usually by treatment or harvest. Prescribed use of EMB between 2012 and 2015 was 242% higher than between 2000 and 2003. In 2014, approval of hydrogen peroxide as a sea lice treatment was required because of apparent reductions in EMB efficacy. In other regions, onset of resistance to EMB is well documented and associated with increased treatment frequency.
The primary goal of this research is to identify the opportunities for reducing EMB treatment frequency in BC, thereby reducing the risk of EMB-resistant sea lice. Sea lice abundances differ among Fish Health Management Zones and understanding the factors contributing to this spatial heterogeneity will better inform the risk of infection and the need to use drugs or pesticides to manage sea lice on Atlantic Salmon farms.
Date: Apr. 2017 – Mar. 2018
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Simon Jones (DFO)
Project Team: Katie Verkaik (DFO); William Sears (U Guelph); Carl Ribble (Centre for Coastal Health/U Calgary)
Contact: Simon.Jones@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/fhtt-etsp-2017-p-01-eng.html
Fish health
Physiological consequences of piscine orthoreovirus (PRV) infection of Atlantic and Pacific salmonids
Piscine orthoreovirus (PRV) type 1 can establish long-term infections in the blood of numerous salmon species and in some instances has been linked to a disease state. The development of high load infections with the potential to cause disease has raised concerns regarding PRV’s impact on salmon physiology and overall fish health.
This project aimed to determine if PRV infections have physiological consequences and whether the virus can directly impact salmon health. The cardiorespiratory performance (swimming ability) of PRV-infected Atlantic and Sockeye Salmon, in comparison to uninfected controls, was evaluated in a laboratory setting. Oxygen binding potential and maximum oxygen consumption were measured and compared between infected and non-infected individuals.
It was found that the potential of oxygen to bind to blood cells and the carrying capacity of blood cells were not affected by PRV. There was, however, severe presence of the virus in the bloodstream, associated minor heart disease, and short-term cell activation of anti-viral response pathways. The acute and chronic PRV infections did not cause sustained differences in the breathing capabilities of the infected fish. Preliminary analyses suggest there are minor physiological impacts of PRV to Sockeye Salmon. The lack of functional harm to salmon infected with PRV implies that pathogen load is not in itself a good predictor of disease.
Date: Jan. 2017 – Mar. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: BC Salmon Farmers Association (BCSFA); Natural Science and Engineering Research Council (NSERC)
Project Leader: Kyle Garver (DFO); Anthony Farrell (UBC)
Project Team: Mark Polinski (DFO); Colin Brauner, Yangfan Zhang, Phillip Morrison (UBC)
Collaborators: Jeremy Dunn (BCSFA)
Contact: Kyle.Garver@dfo-mpo.gc.ca

An overview (top) and a detailed view (bottom) of 8-channel automatic intermittent-flow respirometry system to measure integrated respiratory assessment paradigm (IRAP).
Photo: Yangfan Zhang (UBC)

Blood samples contained in the tonometer (left). The tonometer is then placed in the spectrophotometer (right) to quantify the oxygen equilibrium curve of hemoglobin.
Photo: Phillip R. Morrison (UBC)
Assessment and mitigation of whirling disease, Myxobolus cerebralis, in privately stocked ponds in Alberta
Whirling disease, Myxobolus cerebralis (Mc), was detected in Johnson Lake in Banff National Park in Alberta in 2016. Following a CFIA epidemiological assessment, 684 privately stocked ponds in Alberta were found to be stocked from potentially infected facilities. Currently 94 of the 684 ponds have ’suspended’ licenses, preventing licensees from stocking trout until they have proven to be disease-free, as stocked ponds with established Mc lifecycles may create hotspots in Alberta that can result in wild, native trout populations being infected.
Pond owners and the aquaculture industry in Alberta both have a vested interest in determining the disease status of ponds, as well as how to eradicate disease from infected ponds. Ongoing research on stocked ponds in Alberta will assess whether we can determine if the Mc lifecycle has been established through TAM enumeration, qPCR analysis, and/or sentinel studies. In ponds determined to have established Mc lifecycles, research into methods to break the lifecycle will be conducted, including: (1) leaving ponds fallow of fish; (2) draining, tilling, and allowing ponds to freeze; (3) introducing an aquatic agent to eliminate one obligate host (Tubifex tubifex); or (4) eliminating T. tubifex worms with the introduction of high electrical exposure.
This research will allow the province to confidently assess the disease status of privately stocked ponds and make management decisions on whether ponds can continue to be stocked with trout. Additionally, this research will determine if it is possible to eradicate an established whirling disease life cycle from a stocked pond. This work may provide ground-breaking insights into treatment and control of whirling disease, and allow for the continued stocking and sale of farmed trout in Alberta.
Date: Jan. 2019 – Feb. 2020
Funded by: Government of Alberta
Project Leader: Trish Kelley (Government of Alberta)
Project Team: Laurie Gallagher, Jim Wagner, Steve Jimbo (Government of Alberta)
Collaborators: Greg Goss, Patrick Hanington (U Alberta); Barry Nehring (Fish and Wildlife Colorado); Jim Davies (InnoTech Alberta)
Contact: Trish.kelley@gov.ab.ca
Investigation of piscine reovirus (PRV) in the development of disease
Understanding disease determinants of piscine reovirus (PRV) infection is instrumental in ensuring best management practices are implemented towards safeguarding against the development of disease in salmon aquaculture.
Piscine reovirus is a double stranded RNA virus that is a member of the family Reoviridae. In Norway, the virus has been shown to cause heart and skeletal muscle inflammation (HSMI), an emerging disease in Norwegian-farmed Atlantic Salmon. However, in laboratory studies in Canada utilizing PRV isolated from farmed Atlantic Salmon in British Columbia, no disease was transmitted to naïve fish despite establishing significant PRV infections. Consequently, the pathogenic versus non-pathogenic outcomes associated with PRV infection is puzzling and suggests regional-specific factors are needed to cause disease.
To evaluate the individual contribution of host, pathogen, and environmental factors as determinates in the development of HSMI disease, side-by-side challenge studies will be conducted in Norway and Canada. Through a pairwise comparison of the virulence of multiple strains of PRV in multiple stocks of Atlantic Salmon, it will be determined whether the development of HSMI is specifically linked to PRV strain, a host’s genetics, and/or a particular environment. Ultimately, by comparing equivalent PRV exposure studies in Norway and Canada, this project will determine what triggers are required for the development of HSMI in Atlantic Salmon.
Date: Apr. 2017 – Mar. 2019
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Kyle Garver (DFO)
Project Team: Mark Polinski, Mark Braceland, Nellie Gagné (DFO)
Collaborators: Øystein Wessel, Espen Rimstad (NMBU)
Contact: Kyle.Garver@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/fhtt-etsp-2016-p-03-eng.html
Piscine reovirus (PRV): Characterisation, Atlantic Salmon susceptibility, and initial survey in farmed and wild salmonids in Atlantic Canada
It is important for the industry and for DFO to understand the distribution of the piscine reovirus (PRV), how and if it can affect fish, and to understand the relationship with PRV across salmon producing countries, as strain differences, and fish genetic background influence disease susceptibility.
PRV has been linked to heart and skeletal muscle inflammation (HSMI) in Atlantic Salmon. The role of PRV in HSMI development is not perfectly clear, as PRV is often found in fish without symptoms of HSMI. Although the host range of this virus appears primarily restricted to salmonids, it has been occasionally detected in a few non-salmonid species.
In western North America, where PRV particles in salmon blood are also occasionally detected in some fish, there is no known occurrence of HSMI. This suggests that PRV from western North America is not harmful, or that factors other than the presence of PRV are required to cause the disease. In 2015, Atlantic Salmon from the East Coast were tested and the presence of PRV was detected for the first time. The genetic sequence of the strain of virus found on the East Coast bears a close resemblance to the West Coast strain of PRV. As there are no reports of HSMI-like syndromes in salmon, the significance of PRV detection is unclear.
A survey and characterisation of the local PRV is important in order to know the current distribution of the virus on the East Coast, and the potential for PRV to compromise wild and farmed Atlantic Salmon. This research builds on other studies about PRV. At the moment, PRV has not been found in wild salmonids on the East Coast. Farmed fish in hatcheries and in sea cages have a variable prevalence. PRV-free smolts stocked in sea cages will eventually have a PRV prevalence of 100%, indicating the high infectivity and prevalence of the virus in the environment.
Date: Apr. 2016 – Mar. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd.
Project Leader: Nellie Gagné (DFO)
Project Team: Delphine Ditlecadet, Francis Leblanc, Steven Leadbeater, Philip Byrne (DFO)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-g-01-eng.html
Sampling blood from fish to detect the presence of viruses.
Photo: DFO
Prevalence and transmission dynamics of piscine reovirus (PRV) in the marine environment
Piscine reovirus (PRV) is globally distributed, and has been detected in Atlantic Salmon and nearly all Pacific salmon species. The virulence of PRV, like most reoviruses, appears to be generally low. However, in some instances of commercial salmon aquaculture, PRV has been associated with disease.
On the West coast of Canada, PRV is prevalent in both wild and farmed salmon species. Yet, when, where, and how these fish become infected with this virus remain unclear. Preliminary surveys of farmed Atlantic Salmon suggest that PRV infections predominately appear after the farm fish have been moved to the seawater net-pen, suggesting a marine origin to the source of the infection.
To better understand the infection dynamics of PRV in the marine environment, this project aims to: 1) identify when, where and how long PRV is present in farmed Atlantic Salmon in BC; 2) create a test to sensitively detect PRV from seawater and use this test to screen environmental seawater samples; 3) monitor the amount of virus shed from the fish into seawater following laboratory infection to see how long the virus remains infective; and 4) identify the susceptibility of multiple fish lineages and species in becoming infected with PRV after making physical contact with infected farmed Atlantic Salmon.
In addition, this study aims to quantify the key parameters required to assess PRV transmission and quantify risk of PRV spread from Atlantic Salmon. This knowledge will be directly applicable in establishing best salmon farming practices concerning PRV. Understanding the risks associated with PRV to farmed and wild salmon is highly important to DFO and the salmon farming industry to ensure appropriate management responses are developed.
Start Date: Apr. 2018 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-funded by: Cermaq Canada Ltd.
Project Leader: Kyle Garver (DFO)
Project Team: Mark Polinski, Jon Richard, Haley Matkin, Lynden Gross (DFO)
Collaborators : Barry Milligan (Cermaq Canada Ltd.)
Contact: Kyle.Garver@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-p-01-eng.html
PRV susceptibility of Atlantic Salmon at different life stages and eastern Canadian vs European farmed salmon comparative study
The piscine reovirus (PRV) is a recently identified virus that has been linked to heart and skeletal muscle inflammation (HSMI) in Atlantic Salmon. The actual role of PRV in HSMI development has been difficult to confirm, as PRV is often detected in fish without symptoms of HSMI and viral culture cannot be done. One way of detecting PRV is by examining the blood, spleen and kidneys of fishes, as erythrocytes are major targets for PRV infection.
In Norway, high loads of PRV have been suggested as a requirement for the development of HSMI in Atlantic Salmon. In western North America, PRV is detected in both wild Pacific salmon and farmed Atlantic Salmon, with a high prevalence in farmed fish. In addition, the observation of lesions typical of HSMI by histology has been made recently in one farm in British Columbia, where lesions were more pronounced in individuals with higher loads of PRV. Data gathered over the past two years provide a significant advance into the PRV situation in eastern North America, but some knowledge gaps remain to determine if the detected PRV strains actually pose a threat to wild or farmed Atlantic Salmon, and if preventive management procedures can be developed to reduce the potential risk of HSMI outbreak.
The goal of this work is to further demonstrate that HSMI is unlikely to be an issue on the eastern coast of Canada, to begin to explore the reason for this resistance, and to reduce the chances that HSMI will ever become an issue by providing possible mitigation solutions to the aquaculture industry.
Date: Apr. 2018 – Mar. 2022
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd.
Project Leader: Nellie Gagné (DFO)
Project Team: Delphine Ditlecadet, Francis Leblanc, Philip Byrne (DFO)
Collaborators : Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Nellie.Gagné@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-g-01-eng.html

DFO Scientists performing fish dissections to detect virus in tissues.
Photo: DFO
Pathogen susceptibility of Pacific Salmon – Phase 1: Salmon anemia virus (ISAV) and alphavirus (SAV)
Infectious salmon anemia virus (ISAV) and salmon alphavirus (SAV) are known to affect farmed Atlantic Salmon in various salmon producing countries, but the susceptibility of Sockeye Salmon was unknown. In response to the recommendation that DFO undertake research into the health of Fraser River Sockeye Salmon and their susceptibility to disease of farmed salmon, this research project examined the susceptibility of Sockeye Salmon to these pathogens and the disease transmission potential between Atlantic and Sockeye Salmon.
All experiments were carried out in duplicate tanks, for sampling and observation of mortalities respectively. In each tank, 15 Atlantic Salmon were injected with a viral strain and mixed with 40 naïve Sockeye and 30 naïve Atlantic Salmon. A negative control tank was also set up. An Atlantic (NA) and a European (EU) strain of ISAV were evaluated as well as SAV3. All Atlantic Salmon and few Sockeye Salmon tested positive for ISAV over the 12 week study period. There was one mortality of Sockeye Salmon in the NA strain tank, although ISAV disease was not strongly supported. Mortality in Atlantic Salmon was elevated as expected. After a 12 week exposure to SAV, all Atlantic and no Sockeye Salmon tested positive and no mortality was observed. To verify if Sockeye Salmon exposed to ISAV or SAV were infectious, reciprocal challenges were performed. Naïve Atlantic Salmon were added to the tanks containing the previously exposed Sockeye individuals. The naïve fish were sampled five weeks later to determine if they had become infected. None were found positive for ISAV or SAV.
The results show that Sockeye Salmon passing by aquaculture installations would not be negatively impacted by exposure to these viruses, even in the unlikely eventuality of an outbreak. Both viruses represent a higher risk to the farmed Atlantic Salmon population than to Sockeye Salmon.
The knowledge generated by this project could help facilitate management decisions and the implementation of mitigation measures, if ISAV or SAV was discovered in a region where Sockeye Salmon are present. Additionally, for training and reference purposes, collections of histological slides of SAV-infected tissues were prepared. The virus is exotic to Canada, thus a rapid outbreak response from DFO would be facilitated with access to this reference collection.
Date: Apr. 2014 – Jun. 2018
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Marine Harvest Canada Limited
Project Leader: Nellie Gagné (DFO)
Project Team: Francis Leblanc, Mélanie Roy, Crystal Collette-Belliveau, Delphine Ditlecadet, Philip Byrne, Steven Leadbeater (DFO)
Collaborators: Diane Morrison (Marine Harvest Canada Limited)
Contact: Nellie.Gagne@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/G-14-01-003-eng.html

Fisheries and Oceans Canada's Gulf Biocontainment Unit – Aquatic Animal Health Laboratory is located in Canadian Food Inspection Agency's laboratory in Charlottetown, P.E.I. The lab contributes to research and development, scientific advice, and testing related to high-risk pathogens in saltwater and freshwater.
Video: Aquatic Animal Health Laboratory (DFO)

Fish holding tank in quarantine, Gulf Biocontainment Unit, Charlottetown, PE.
Photo: Nellie Gagné (DFO)

Atlantic Salmon smolts.
Photo: Nellie Gagné (DFO)
Salmon gill poxvirus-like (SGPV-like): characterization, Atlantic Salmon susceptibility, and initial survey in farmed and wild salmon
Poxviruses are large DNA viruses of vertebrates and insects causing disease in many animal species. In the spring of 2006, a new poxvirus, the salmon gill poxvirus (SGPV), was discovered on the gills of salmon suffering from proliferative gill disease (PGD) in freshwater in northern Norway. Later the same year, this virus was also found on salmon gills at two marine sites in western Norway, where all farms suffered high losses associated with the presence of this virus. Clinical disease symptoms are lethargy, respiratory distress, and mortality.
In 2015, an unknown virus was isolated from tissues sampled from a wild Atlantic Salmon caught in a river in New Brunswick. It was found that this virus was “SGPV-like” since Next Generation sequencing showed 80–90% similarity to Norwegian SGPV. The implications of this finding are, however, unclear.
The objectives of the current project are to characterise SGPV-like sequences from Canada’s East coast, describe their current and historical geographic distributions and compare their genotypes to known strains of SGPV. Assays suitable for the screening of local strains will also be developed.
Through the ongoing survey, we detected SGPV in wild and farmed salmonids, at relatively low prevalence and low levels of infection. Attempts to infect Atlantic Salmon with the isolated virus were unsuccessful, indicating that secondary factors are required for the infection. There are two distinct clades of SGPV present in Atlantic Canada. SGPV from the East coast is not linked to any pathology. However, the virus is causing increasingly important mortalities in hatcheries and on sea farms in Europe. Understanding the genetic factors, viral and host related, and the risk posed by this emerging pathogen is important.
Date: Apr. 2016 – Mar. 2019
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd.
Project Leader: Nellie Gagné (DFO)
Project Team: Delphine Ditlecadet, Valérie Godbout, Jean-René Arseneau, Francis Leblanc, Steven Leadbeater, Philip Byrne (DFO)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Nellie.Gagne@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-G-02-eng.html
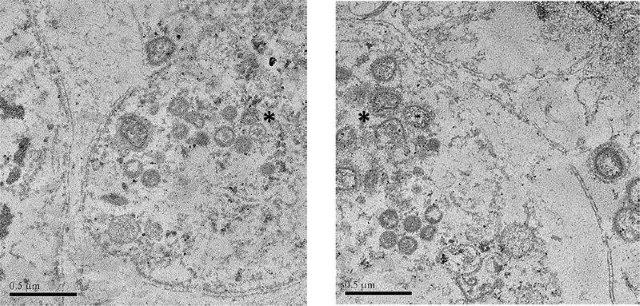
Transmission electron microscopy image of CHSE-214 cells infected with salmon gill poxvirus from Atlantic Canada.
Photo: University of New Brunswick, Microscopy and Microanalysis Facility, Fredericton
Salmon gill poxvirus (SGPV) characterisation in farmed and wild salmon — Phase II
Poxviruses are large DNA viruses of vertebrates and insects causing disease in many animal species. In the spring of 2006, a new poxvirus, salmon gill poxvirus (SGPV), was identified by electron microscopy in the gills of salmon suffering from proliferative gill disease (PGD) in freshwater in northern and western Norway. Clinical disease symptoms are lethargy, respiratory distress, and mortality. A recent survey of SGPV in wild and farmed Atlantic Salmon in Norway revealed a high prevalence of the virus, with high virus loads reported in clinically diseased fish. In Canada, an SGPV-like virus was recently identified and isolated from a wild Atlantic Salmon caught in a river in New Brunswick. It was also reported that salmon gill diseases of unknown etiology were detected on the East coast of Canada.
Data gathered through a previous project funded by DFO’s Aquaculture Collaborative Research and Development Program (16-1-G-02) are a significant step forward to better understand the SGPV situation in eastern North America, but more work is needed to understand its prevalence in hatcheries and the actual threat SGPV may represent for Atlantic Salmon.
This project will provide more in-depth knowledge of SGPV-like virus on the East coast. Specifically, the project will conduct exhaustive screening of SGPV in broods and in hatcheries, determine if SGPV can be detected in incoming waters and determine if more viruses can be obtained through cell culture. Moreover, the project aims to determine if infected salmon can infect naïve salmon, confirm that gills are the target tissue of the SGPV-like variants and look for signs of clinical disease in fish. Finally, the susceptibility/resistance to these viruses of the salmon stocks used in Atlantic Canada will be assessed.
Date: Apr. 2018 – Mar. 2022
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd.
Project Leader: Nellie Gagné (DFO)
Project Team: Delphine Ditlecadet, Francis Leblanc, Philip Byrne, Steven Leadbeater (DFO)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Nellie.Gagne@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-g-02-eng.html

Steve Leadbeater (front) and Francis Leblanc, fish tanks in quarantine at St. Andrews Biological Station.
Photo: DFO
Validation of pooling tissues for pathogen detection (infectious salmon anemia virus and Haplosporidium nelsoni-MSX)
The DFO’s National Aquatic Animal Health Laboratories provide diagnostic testing services to clients, for example CFIA. Pooling tissues for diagnostics is advantageous in time and cost, as more animals can be tested at the same time. However, the impact of pooling on diagnostic sensitivity must be determined for molecular-based methods. The ability to detect one infected animal, in a pool of non-infected animals might be reduced after pooling, versus in individual detections. But, the possibility of testing more individuals can offset by this impact.
This project aims to establish a pooling process that is efficient for high-throughput diagnostic labs. We will also examine the repeatability and impact of pooling three animal tissues, when only one of those is infected. Pools will be tested in triplicate by analysts using established quantitative Polymerase Chain Reaction (qPCR) methods, and the cycle threshold (Ct) of the pooled samples will be compared to the value obtained by testing the tissue individually. This will be done for two pathogens where assays were validated for single tissues, i.e., infectious salmon anemia (ISAV) and Haplosporidium nelsoni (MSX). Pooled diagnostic sensitivity will be determined for these two assays.
Although the theoretical impact of pooling is known, it is important to validate this change in sample processing, so that clients are informed of the impact pooling represents on diagnostic sensitivity. With pools of three, we anticipate that the risk of a false negative detection will be minimal, and acceptable.
Date: Apr. 2018 – Mar. 2019
Funded by: DFO – Centre for Aquatic Animal Health Research and Diagnostic (DFO – CAAHRD)
Project Leader: Nellie Gagné (DFO)
Project Team: Jean-René Arseneau (DFO)
Contact: Nellie.Gagne@dfo-mpo.gc.ca

Jean-René Arseneau, research analyst at DFO Gulf Fisheries Centre, Moncton, prepares samples for molecular testing.
Photo: Nellie Gagné (DFO)
The effect of dietary camelina oil on health of salmon
Traditional salmon feeds use high levels of fishmeal and fish oil to meet the nutritional needs of the fish, but these ingredients face large fluctuations in price and availability. Lower-cost alternatives have been investigated, including canola oil, soy oil and poultry fat. Oil from the plant species Camelina sativa is another promising option. However, there are no data available on vegetable oil’s health effects on Chinook Salmon.
Trials were conducted to evaluate the impact of replacing dietary fish oil with camelina oil on the health and growth of Chinook Salmon in seawater. A wide range of substitution levels was examined, as well as a comparison between camelina oil and canola oil.
The results of this study show that camelina oil can replace fish oil in diets for juvenile Chinook Salmon with no lasting impact on fish health (i.e., inflammation of the gut). Replacement of dietary fish oil by camelina oil also did not affect growth or feed efficiency in fish as large as one kilogram. The effect of canola oil was generally similar to camelina oil, but survival was slightly reduced in the fish fed the canola diets and there was a significant difference between these two oils on muscle fatty acid profile. The fatty acid profiles of fish fed with camelina oil could be beneficial to human health, relative to those fed with canola oil.
The results of this project indicated that camelina oil can replace fish oil in Chinook Salmon diets with no lasting impact on fish health or growth, at least on a short term basis.
Date: Sep. 2015 – Oct. 2016
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Creative Salmon Co. Ltd.
Project Leader: Ian Forster (DFO)
Project Team: Simon Jones (DFO); Marije Booman (UVic); Barb Cannon, Tim Rundle (Creative Salmon Co. Ltd.); Jack Grushcow (Linnaeus Plant Sciences Inc.); Brad Hicks (Taplow Ventures Ltd.)
Collaborators: Barb Cannon, Tim Rundle (Creative Salmon Co. Ltd.)
Contact: Ian.Forster@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-2-P-03-eng.html

Salmon in a fiberglass tank at the culture facility.
Photo: Ian Forster (DFO)
Viral hemorrhagic septicaemia virus (VHSV) evolution and adaptation potential in farmed Atlantic Salmon in British Columbia
Viral hemorrhagic septicemia virus (VHSV) occurs in British Columbia’s Atlantic Salmon aquaculture sea-cage industry. Molecular epidemiological studies have demonstrated that the source of VHSV in farmed fish is often a result of “spillover” from a wild reservoir, likely from infected Pacific Herring and Sardine. To date, the detection of VHSV in farmed salmon has yet to be associated with significant disease. Nevertheless, with the annual finding of VHSV within Atlantic Salmon and VHSVs potential for adaptation, concerns arise from the ability of the naturally occurring marine VHSV to adapt and become pathogenic to farmed Atlantic Salmon.
This project will compare VHSV isolates obtained from Pacific Herring and Atlantic Salmon infected under controlled laboratory conditions to characterize the viral mutant spectrum present in infected fish and identify the mechanisms or genetic background facilitating inter-species transmission of VHSV.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Kyle Garver (DFO)
Project Team: Jon Richard, Laura Hawley, Lynden Gross, Haley Matkin (DFO)
Contact: Kyle.Garver@dfo-mpo.gc.ca
Website: https://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-p-02-eng.html
Marine reservoirs of infectious agents associated with proliferative gill disorders in farmed salmon
Gill diseases contribute to economically important production losses in Atlantic Salmon aquaculture. This project will improve our understanding of reservoirs of infections with infectious agents associated with these disorders. One of these, amoebic gill disease (AGD), was diagnosed for the first time in BC in 2014 and the causative agent, Paramoeba perurans, has been detected in BC. Another agent that is associated with proliferative gill inflammation (PGI) in Europe, the microsporidian, Desmozoon lepeophtherii, has been detected in BC and Washington State. Several cases of a PGI-like condition were reported in farmed salmon in BC in 2015. This project capitalizes on an opportunity to benefit from a unique set of samples; juvenile salmon collected by industry as part of their sea lice certification program. Specifically, these samples will be examined for evidence of their involvement as reservoirs of infection with agents associated with proliferative gill disorders in farmed salmon.
The objectives of this project are to:
- determine distribution of P. perurans and D. lepeophtherii in wild Pacific salmon and salmon lice collected in proximity to marine netpens;
- describe the occurrence of proliferative gill lesions in wild fish;
- characterize the genomic sequence of BC variants of P. perurans and D. lepeophtherii; and,
- conduct laboratory transmission studies to identify and quantify host and environmental parameters surrounding transmission of causative agents between candidate reservoir species and Atlantic Salmon.
These objectives reflect the knowledge gaps concerning PGI and causal agents in farmed salmon in BC. The key anticipated project outcome will be knowledge related to the distribution and causes of PGI in BC. This knowledge will inform further development of farmed fish health management strategies.
Date: Apr. 2016 – Mar. 2020
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR); DFO – Aquaculture Collaborative Research and Development (DFO – ACRDP); BC Salmon Farmers Association – Marine Environmental Research Program (BCSFA – MERP)
Project Leader: Simon Jones (DFO)
Project Team: Ashley Burton (DFO); Gary Marty (AHC)
Collaborators: Mykolas Kamaitis (Marine Harvest Canada Ltd.); Tim Hewison (Grieg Seafoods BC); Barry Milligan (Cermaq Canada)
Contact: Simon.Jones@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-P-01-eng.html
Canada research chair in fish and environmental immunology
Aquaculture now provides over 50% of the annual fish consumption globally, but fish are cold-blooded and thus the ability of their immune systems to resist disease is affected by the environmental temperature fluctuations and the long-term increases that climate change will bring.
Dr. Dixon’s laboratory will develop and use state-of-the-art immunoassays and cell biology techniques to determine the immune status of fish to help develop next-generation vaccines that will provide disease protection. The effect of both high and low environmental temperature on immune function will be investigated, as well as the effects of gender on immune responses. This research will produce key immune knowledge required to make aquaculture vaccines more effective and will investigate effects of sex reversal on immune function of aquaculture species. The knowledge and diagnostic assays developed during this CRC project will prevent disease losses, saving billions of dollars and providing a stable food source for the world’s population.
Date: Oct. 2018 – Oct. 2025
Funded by: Natural Sciences and Engineering Research Council (NSERC) – Canada Research Chairs Program
Project Leader: Brian Dixon (U Waterloo)
Collaborators: Yellow Island Aquaculture
Contact: bdixon@waterloo.ca
Validation of genomic selection among Atlantic Salmon for resistance to infection by sea lice (Lepeophtheirus salmonis), and concomitant susceptibility to infectious salmon anemia virus (ISAV)
The sea louse, Lepeoptheirus salmonis, continues to cause the greatest impact on the economics of salmon farming, globally. It is generally believed that selection of farmed salmon for sea louse resistance, in combination with the use of a vaccination, would be the most environmentally-friendly approach to sea lice management. However, there are no effective sea louse vaccines available at this time. As such, it is imperative for the industry to assess its genetic stocks for their robustness against sea lice disease.
Despite the benefits that selecting for one resistance trait may provide, there is always the concern that gains in resistance to one trait may impact another trait. This could result in gains or losses in resistance, growth rate or unforeseen traits. While a previous project funded by DFO’s Aquaculture Collaborative Research and Development Program (ACRDP 15-1-M-01) has produced selection information for sea lice resistance, it is not known what impact breeding for resistance to sea lice would have on the susceptibility to other pathogens, such as infectious salmon anemia virus (ISAV). ISAV is considered a major pathogen of Atlantic Salmon on the Eastern coast of Canada and, despite much improved management practices to minimize outbreaks, ISAV continues to cause losses and force eradication events that impact the aquaculture industry of Atlantic Canada.
In this study, groups of salmon, including presumed sea lice resistant families, will be exposed to sea lice to assess gains in sea lice resistance. The same mix of salmon families will be challenged with ISAV in order to determine whether selection for sea lice resistance has any impact on resistance to ISAV.
Date: Apr. 2018 – Mar. 2021
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Kelly Cove Salmon Ltd.
Project Leader: Steven Leadbeater (DFO)
Project Team: Marc Trudel (DFO); Jake Elliott, Frank Powell (Cooke Aquaculture Inc.); Anthony Manning (RPC)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Steven.Leadbeater@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-m-01-eng.html
Biofouling communities on Atlantic Salmon (Salmo salar) farm nets: Exploring links to fish gill health
Biofouling is a widely recognized problem within the finfish aquaculture industry. It can lead to compromised cage structure, lower flow rates within cages, and drops in dissolved oxygen concentration, which may ultimately lead to impacts on fish health. Management practices at fish farms mainly involve regular physical removal of the organisms by in situ net cleaning. In recent years, net cleaning strategies have evolved into using automated power-washing machines with high pressure (3200 psi) water jets, which create suspended biotic debris. This biological particulate material has the potential to affect salmon health by damaging the fish gills. Gill disorders are a common occurrence at fish farms, however, they are usually associated with viruses, bacteria, parasites, phytoplankton blooms, and zooplankton (cnidarian) swarms. There has been little investigation into the potential effect of biofouling organisms on the health of cultured fish.
This project will determine the biofouling communities (species and quantities) at three Atlantic Salmon (Salmo salar) farm sites off the coast of Vancouver Island. Five replicates (one per each of five pens) of 30 x 30 cm net pieces will be installed at two depths (one and five meters) and on two net types (predator and containment) and removed monthly at each site for a year. Fish-gill health will be investigated through gill scores taken from fish in one pen per site every month. Phytoplankton and zooplankton communities will also be examined daily and bi-weekly, respectively. The resultant data will be analysed to determine if there is a correlation between poor gill heath and certain species of biofouling/pelagic organisms.
The project will provide industry with insights into the impacts of biofouling, zooplankton, and phytoplankton species on fish-gill health, which will allow appropriate mitigation measures to be put in place to ensure the health of the fish.
Date: May 2018 – Apr. 2020
Funded by: Grieg Seafood BC Ltd.
Co-Funded by: Fisheries and Oceans Canada (DFO); University of Victoria (U Vic)
Project Leader: Chris Pearce (DFO)
Project Team: Steve Cross, Mark Flaherty, Raquel Greiter (U Vic)
Collaborators: Bogdan Vornicu, Liam Peck, Patrick Whittaker, Tim Hewison, Matthew Patterson (Grieg Seafood BC Ltd.); Nicky Haigh (Microthalassia Consultants)
Contact: Chris.Pearce@dfo-mpo.gc.ca
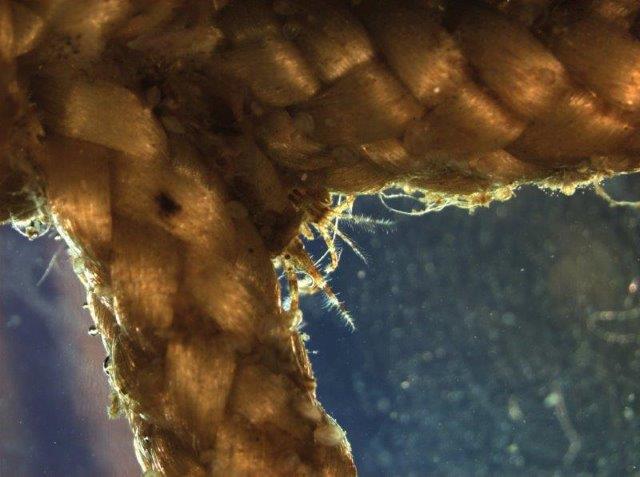
Two tube-dwelling sea fleas (Ericthonius spp.) hiding under a net sample from an Atlantic Salmon (Salmo salar) farm off the Sunshine Coast.
Photo: Raquel Greiter Loerzer (U Vic)
Screening of cultured Atlantic Salmon for resistance and susceptibility to infection by sea lice (Lepeoptheirus salmonis) and Renibacterium salmoninarum, the causative agent of bacterial kidney disease (BKD)
While vaccination is one approach to impart disease resistance, there is significant variation among Atlantic Salmon in terms of vaccine responsiveness. It is imperative to assess the genetic stocks of salmon for their robustness against disease.
This research project will test family crosses of farmed salmon for disease resistance to Lepeoptheirus salmonis (sea lice) and Renibacterium salmoninarum (the causative agent of bacterial kidney disease, BKD). Testing of more than one disease agent (in this case, sea lice and the causative agent of BKD) will help assess what resistance to one agent type may mean for resistance to another common agent. Results from this project will contribute to research that will aid in broodstock selection, enhancing resistance of farmed fish to both sea lice and BKD infections, with the on-going goal of reducing farmed fish losses and improving the sustainability of the Canadian aquaculture industry.
Start Date: Sep. 2015 – Jun. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO– ACRDP)
Co-funded by: Kelly Cove Salmon Ltd.
Project Leader: Steven Leadbeater (DFO)
Project Team: Keng Pee Ang (Kelly Cove Salmon Ltd.); J.A.K. Jake Elliott, Frank Powell (Cooke Aquaculture Inc.); Anthony J. Manning (RPC)
Collaborators : Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Steven.Leadbeater@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-M-01-eng.html

Sea lice colour correction.
Photo: Cindy Hawthorne (DFO)

Melissa Holborne, Gregor Reid and Steve Leadbeater Sea lice counting.
Photo: Cindy Hawthorne (DFO)
Characteristics of British Columbia isolates of Piscirickettsia salmonis relevant for understanding pathogen transmission and infectivity
The bacterium Piscirickettsia salmonis is the causative agent of salmonid rickettsial septicaemia (SRS), a disease in marine farmed salmon with significant outbreaks in Europe, Chile and Canada. In British Columbia, the causative agent P. salmonis has been detected in wild Pink and Chinook Salmon and in farmed Atlantic Salmon, but the disease (SRS) has not been reported in wild salmon. Infections were originally detected at farm sites in southwestern Vancouver Island, but since 2015, the range has expanded northward to include the Sunshine Coast, Discovery Islands, Broughton Archipelago and northwestern Vancouver Island. Between 2002 and 2016, 36 fish health events associated with P. salmonis were reported at Atlantic Salmon farms in BC. (A fish health event is defined as “a suspected or active disease occurrence within an aquaculture facility that requires the involvement of a veterinarian and any measure that is intended to reduce or mitigate impact and risk that is associated with that occurrence or event.”)
Concern regarding pathogen transfer from farmed fish to wild Sockeye Salmon in the Discovery Islands necessitates a risk assessment analysis for P. salmonis. This project will address the many knowledge gaps needed in the pending risk assessment and will inform both Aquaculture Management and aquaculture industry of the nature of this disease. Studies will focus on factors influencing survival of the bacterium in the marine environment, susceptibility of Sockeye Salmon to P. salmonis, the minimum infectious dose for Atlantic, Pink and Sockeye Salmon, progression of disease, host response to infection, and the bacterial shedding rate from infected salmon.
Date: Apr. 2018 – Mar. 2019
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Simon Jones (DFO)
Project Team: Amy Long (DFO)
Contact: Simon.Jones@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/fhtt-etsp-2018-p-01-eng.html
Integrated pathogen management of co-infection in Atlantic Salmon
At Atlantic Salmon farms, it is not uncommon for fish to be infected with multiple pathogens, including sea lice, bacteria and viruses. Such co-infections are very complicated to assess and they can cause severe economic losses for aquaculture farmers and the industry. Relatively little research has been conducted on co-infections in salmon because it requires complex experimental designs and facilities.
This research team is using functional genomics tools and techniques, including DNA microarrays, RNA sequencing, and both singleplex and multiplex qPCR, to identify genes and molecular mechanisms involved in salmon responses to co-infections. Atlantic Salmon trials use co-infection/co-stimulation with sea lice, and then antigen or bacteria or virus to generate genomics derived biomarkers (i.e., genes that respond to co-infection). Biomarkers combined with fish performance and other complementary data (e.g., pathogen load, lipid/fatty acid profiles, histology) will be analyzed to determine the optimal clinical diet to treat the co-infection phenotype. Development of Atlantic Salmon feeds to combat co-infections can reduce the need for chemical and pharmaceutical interventions and ultimately improve farmed salmon health.
Research is ongoing, but current pathogens being studied include those that cause ISA, SRS and winter ulcer in co-infection scenarios with sea lice. So far, a commonality between studies is the significant impact co-infection has on the host transcriptome and survival outcome compared to single infections. Furthermore, successful functional feed intervention differs significantly between single and co-infection of the same pathogens. Individual biomarkers of these outcomes have been identified and are being further pursued.
Date: Oct. 2016 – Sep. 2019
Funded by: Genome Canada — Genome Applications Partnership Program
Co-funded by: Cargill Animal Nutrition; InnovateNL; Mitacs; UPEI; Genome Atlantic
Project Leader: Matthew Rise (MUN), Richard Taylor (Cargill Animal Nutrition)
Project Team: Mark Fast, Laura Braden, Sarah Purcell, Shona Whyte, Dylan Michaud, Kathleen Parrish, Laura Carvalho (UPEI); Christopher Parrish, Albert Caballero-Solares, Umasuthan Navaneethaiyer, Xi Xue, Tomer Katan, Amir Jalali, Mohamed Emam (MUN); Ragna Heggebo, Stanko Skugor, Eva Jakob, Jorge Pino (Cargill)
Collaborators: Jillian Westcott (MUN), Barbara Nowak (U of Tasmania), Barry Milligan (Cermaq)
Contact: mrise@mun.ca; richard_taylor@cargill.com

Members of the IPMC project team in the Hoplite Research Lab, AVC-UPEI.
Photo: UPEI
Dr. Albert Caballero-Solares counting sea lice copepodids, MUN-CDRF.
Photo: Dr. Umasuthan Navaneethaiyer (MUN)

Xi Xue counting sea lice copepodids, MUN-CDRF.
Photo: Dr. Jillian Westcott (MUN)

Dr. Umasuthan Navaneethaiyer setting up PCRs, Rise lab, MUN
Photo: Xi Xue (MUN)
Occurrence, distribution and causes of gill disease in salmonids in British Columbia
Gill diseases and disorders among Atlantic Salmon raised in seawater net pens are an emergent and important cause of losses to industry. There is a need to better describe the causes, distribution, and possible control of gill diseases, which have been attributed to infections with parasites, bacteria, viruses, as well as exposure to algal blooms, jellyfish, and other non-infectious agents.
Gill samples from farmed salmon had microscopic evidence of disease and infection with parasites. The magnitude of disease and infection depended both on the location of the farm and on the time of sampling. In contrast, although very few wild salmon had microscopic evidence of gill disease, parasite infections were observed. Despite the novel occurrence in BC of amoebic gill disease (AGD) immediately prior to the onset of this project, no farm samples in the project had evidence of AGD. Of the two parasites frequently detected in wild and farmed salmon (Desmozoon lepeophtherii and Ichthyobodo salmonis), the latter is a first report from British Columbia. The severity of D. lepeophtherii was increased in samples displaying acute clinical gill disease. A novel gill virus was detected at two farms.
There are two main conclusions of this project: the extent of gill disease in farmed salmon in BC varies by farm and over time, and infections with the same parasites occur in gills of farmed and wild salmon. The knowledge gathered from this project will eventually assist in the recognition, quantification and, ultimately, the management of proliferative gill disease in farmed salmon.
Date: Apr. 2016 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Marine Harvest Canada; Grieg Seafoods BC Ltd.; Cermaq Canada Ltd.
Project Leader: Simon Jones (DFO)
Project Team: Gary Marty (BC Ministry of Agriculture); Kyle Garver, Jackie King, Chrys-Ellen M. Neville, Amy Long, Amelia Mahony (DFO)
Collaborators: Diane Morrison (Marine Harvest Canada); Tim Hewison (Grieg Seafoods BC Ltd.); Barry Milligan (Cermaq Canada Ltd.)
Contact: simon.jones@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-p-04-eng.html; http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-P-02-eng.html
Isolation and identification of bacteria associated with ulcer disease from Atlantic Salmon aquaculture sites in New Brunswick
Ulcer disease is not a monolithic infection but occurs from several known causative bacterial agents. Identification and characterization of local bacterial species and isolates will ensure effective treatment options are available for Canadian Atlantic Salmon producers.
Ulcer causing diseases, such as winter-ulcer, cold-water vibriosis and tenacibaculosis infections, continue to be a problem in Atlantic Salmon aquaculture. Huntsman recently initiated a project to collect and identify bacterial isolates from Atlantic Salmon displaying ulcers from regional culture operations. Freshly dead or moribund fish with ulcers are collected. Then, bacterial culture attempts are made from skin lesions and internal organs on marine agar (MA) and Flexibacter maritimus (FMM) media. Tissue samples are also preserved either by flash freezing in liquid nitrogen then stored in ultra-low temperature conditions or in neutral buffered formaldehyde for possible further analysis. Isolated bacteria will be identified by full 16s ribosomal sequencing and stored for future use as required. Characterization of confirmed infectious bacterial isolates will include sensitivity to a series of antibiotic disks, such as ampicillin, florfenicol, oxytetracycline, streptomycin, etc.
To date, the species of bacteria that have been isolated and are associated with ulcer related disease in fish include Aliivibrio salmonicida, Aliivibrio wodanis, Tenacibaculum marimitus and Vibrio lentus. Key next steps will involve single species or species complex infection model development using collected isolates to further oral treatment, autogenous vaccine trial, or broodstock selection research.
Date: Apr. 2018 – Jun. 2021
Funded by: New Brunswick Innovation Foundation – New Brunswick Innovation Research Chair Program
Project Leader: Ehab Misk (HMSC)
Project Team: Anne McCarthy, Chris Bridger (HMSC)
Contact: ehab.misk@huntsmanmarine.ca
Website: Huntsmanmarine.ca
Mouthrot in Atlantic Salmon
Mouthrot associated with Tenacibaculum maritimum is a major health and welfare problem in farmed Atlantic Salmon smolts in BC, and results in large economic losses. This disease is the main reason antibiotics are used in the region. Affected smolts die with very little external or internal clinical signs other than characteristic small yellow plaques in the mouth. The main objective of this industrial PhD was to gain more knowledge about the disease and to make steps towards developing a vaccine.
Genotyping of BC T. maritimum isolates collected from natural outbreaks of mouthrot showed the presence of two genetic strains that are most closely related to strains from lumpsuckers and Atlantic Salmon in Norway, as well as Atlantic Salmon in Chile. Representative isolates were used to develop a bath challenge model using Atlantic Salmon smolts. T. maritimum was demonstrated to be the causative agent of mouthrot in BC without the need for other stressors or co-infections.
The main pathology in mouthrot is similar to periodontal disease in mammals. The pathological changes are focal, severe, and occur very rapidly with little associated inflammation. A cohabitation experiment showed that T. maritimum readily transfers from infected smolts to naïve ones.
Whole cell inactivated adjuvanted vaccines were created and tested using the developed challenge model. Despite giving an antibody response in immunised fish, the vaccines did not protect the smolts against mouthrot induced through a bath infection. Future research needs to focus on preventative tools, including other types of vaccines such as immersion or live-attenuated.
Date: Dec. 2015 – Dec. 2018
Funded by: Norwegian Research Council
Co-Funded by: Cermaq Canada; Pharmaq AS
Project Leader: Kathleen Frisch (Cermaq Canada)
Project Team: Henrik Duesund, Øyvind Brevik, Sverre Småge (Cermaq Group AS); Are Nylund (U Bergen)
Collaborators: Are Klevan, Rolf Olsen (Pharmaq AS)
Contact: kathleen.frisch@cermaq.com
Website: www.Cermaq.com; www.UIB.no
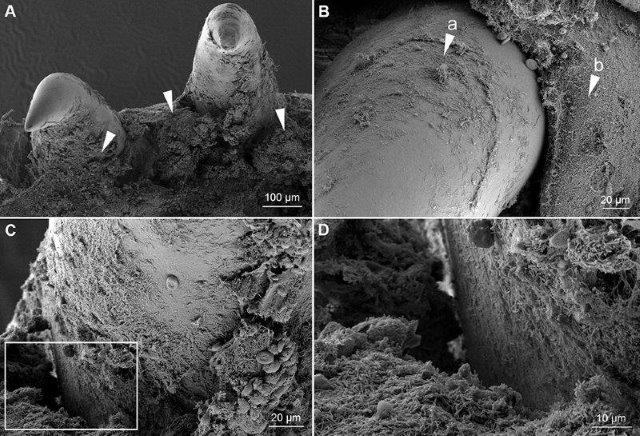
Micrographs of teeth and the surrounding tissue from the mouth of a diseased smolt bath infected with TmarCan15-1 in the cohabitation experiment. (A) Teeth and surrounding gingiva are covered by mats of bacteria with Tenacibaculum maritimum morphology (arrowheads) and the associated tissue is damaged. (B) Zoomed in view of a tooth showing bacterial growth on the surface of the tooth (arrow “a”) as well as the surrounding gingival tissue (arrow “b”). (C) The dentin-enameloid interface with associated tissue destruction. White box indicates area in D. (D) Cellular debris within the bacterial mats.
Photo: Frisch et al.: https://doi.org/10.1371/journal.pone.0206951
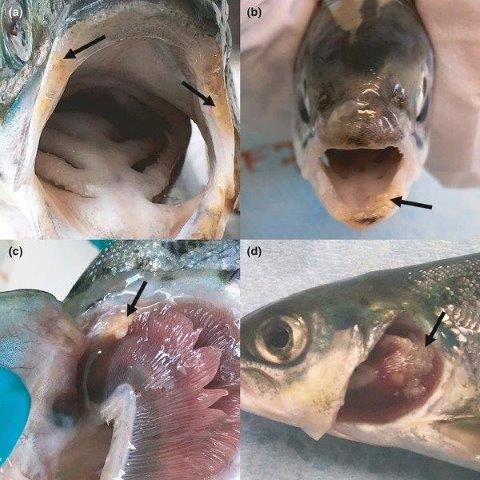
(a) Mouth lesion showing the typical yellow plaque, and (c) gill lesion from a mouthrot-affected fish from a farm in British Columbia, Canada (b) Mouth lesion and (d) gill lesion from a diseased fish in the challenge experiments.
Photo: Frisch et al.: https://doi.org/10.1111/jfd.12818
Investigations into ulcerative skin disease agents, Moritella viscosa and Tenacibaculum spp. In Atlantic Salmon: interactions and in Vivo challenge development
This project examines two pathogens of concern to the salmon farming industry, Moritella viscosa and Tenacibaculum spp., and their roles in skin ulcerative diseases. Recent experience in developing immersion challenge models will be applied to the further understanding of skin ulcerative diseases. The primary focus is to study the bacterial species of the genus Tenacibaculum which causes a condition called “mouthrot” or tenacibaculosis. The secondary focus is to study interactions among bacteria associated with skin ulcers. The project focuses on isolates found on the East coast of Canada as well as interesting changes in the range of environmental temperatures at which pathogenicity is observed in the field (e.g., M. viscosa).
The project develops a basis for progress towards developing vaccines for use in Canada, which will provide the aquaculture industry with an alternate and proactive strategy for dealing with bacterial disease. Vaccines will improve animal welfare and the sustainability of salmon aquaculture while reducing economic loss from fish mortality, antibiotic use, and the downgrading of market product. Results of the study can be used by the aquaculture industry for research on genetic selection for disease resistance, help the fish health community apply better diagnostic methodology for confirming and treating ulcerative skin diseases, and improve understanding of naturally occurring diseases that affect wild salmon and their effects on farmed fish.
Date: Jun. 2016 – Sep. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cooke Aquaculture Inc.
Project Leader: Steven Leadbeater (DFO)
Project Team: Allison MacKinnon (Elanco Canada Ltd.); Anthony J. Manning (RPC)
Collaborators: Leighanne Hawkins (Cooke Aquaculture Inc.)
Contact: Steven.Leadbeater@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-M-02-eng.html
Nucleo-cytoplasmic large DNA viruses of wild Lake Sturgeon (Acipenser fulvescens) in central Canada
Namao virus (NV) is a sturgeon nucleo-cytoplasmic large DNA virus (sNCLDV) that can cause a lethal disease of the integumentary system in cultured Lake Sturgeon. As a group, the sNCLDV are members of the Mimiviridae with CroV as their closest extant virus relative. In this study, the spatial, temporal and genetic patterns of sNCLDV were evaluated for the first time for wild Lake Sturgeon from rivers in central Canada.
A total of 1328 pectoral fin biopsies were collected between 2010 and 2015. Quantitative PCR results with the Q2 test indicated that the virus was endemic in sturgeon of the Hudson Bay drainage basin, with 23.7% of the fish testing positive. The sNCLDV-positive samples were from endangered populations in the Saskatchewan-Nelson River watersheds where virus was detected in 3 to 58% of the sturgeon tested. The highest virus loads were observed in the Nelson River populations in northern Manitoba.
Repeat testing of 26 captured-recaptured individuals revealed temporal heterogeneity with respect to their virus status. Analyses of samples collected annually from the Landing River population revealed that virus presence was inversely correlated with sturgeon age, weight and the number of times sturgeon were handled (as part of the sturgeon monitoring program) prior to virus sample collection. These results suggest that NV infection may reduce wild Lake Sturgeon fitness and survival. Genetic typing of 114 virus isolates indicated that the NV genogroup was dominant in the Hudson Bay drainage basin.
Understanding the ecology of sNCLDV and their potential impact on wild Lake Sturgeon will be integral to developing conservation management plans if these endangered populations are eventually listed on Schedule 1 of the Species at Risk Act. The results of this study can be used to inform disease management strategies for Lake Sturgeon conservation, management and recovery programs. The goal now is to publish the work in a peer-reviewed journal.
Date: May 2010 – Dec. 2018
Funded by: Manitoba Hydro
Project Leader: Sharon Clouthier (DFO)
Project Team: Elissa VanWalleghem, Tamara Schroeder (DFO)
Collaborators: Carol McClure (AquaEpi Research); Don MacDonald (Province of MB); Eric Anderson (Independent researcher); Amanda Caskenette (DFO)
Contact: Sharon.Clouthier@dfo-mpo.gc.ca
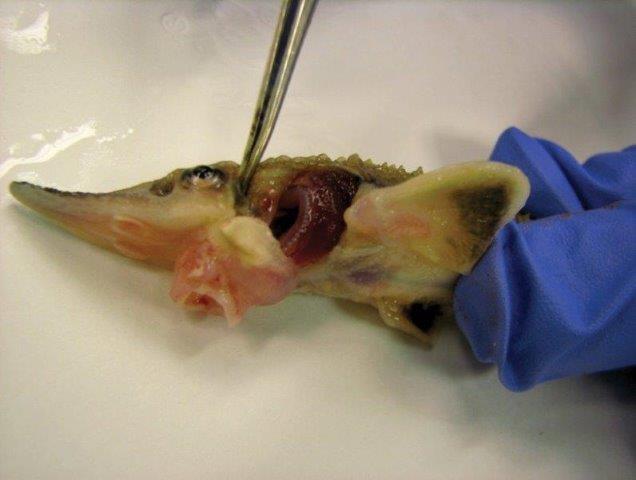
Petechial hemorrhaging is observed on the mouth and snout of a juvenile lake sturgeon from a population experiencing a mortality event associated with namao virus.
Photo: Sharon Clouthier (DFO)
Cellular effectors involved in protective immunity against Kudoa thyrsites in Atlantic Salmon
The histozoic myxozoan parasite Kudoa thyrsites is the causative agent of post-mortem myoliquefaction in post-harvest Atlantic Salmon farmed in British Columbia. The industry in BC incurs substantial economic loss because of Kudoa-associated changes to fillet quality and the impact on consumer acceptance.
This present work followed up on an earlier study that demonstrated there was a protective effect of prior exposure towards development of infection upon subsequent exposure. Specifically, this study looked to characterize the immune response during infection, recovery and re-exposure of Atlantic Salmon to K. thyrsites. By using a multi-pronged approach combining histology, immunohistochemistry, and transcriptomics, this study demonstrated that acquired protective immunity was strongly associated with the presence of MHIIβ+ cells in infected muscle tissue, and furthermore, that these cells were actively involved in detection of the plasmodium and dissemination of myxospores. Moreover, dual-labelling indicated a majority of these cells were CD8α+. These results indicate a cell-mediated immune response is involved in the resolution of K. thyrsites infection in Atlantic Salmon, and further, that CD8α+ cytotoxic T-cell killing is a candidate mechanism for protection upon re-exposure to the parasite.
Data provided by this study supports the role of an antigen-presenting cell population in the protective immune response against K. thyrsites.
Date: Jun. 2013 – Jan. 2017
Funded by: Elanco Animal Health
Co-Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP); Natural Science and Engineering Research Council of Canada (NSERC); Marine Harvest Canada Ltd.
Project Leader: Mark Fast (AVC)
Project Team: Laura Braden, Shona Whyte, Sara Purcell (UPEI); Karina Rasmussen (U Southern Danemark); Lauren Ellis, Amelia Mahony, Steven Cho, Simon Jones (DFO)
Contact: lbraden@upei.ca

Photomicrographs of Kudoa thyrsites-infected muscle tissue probed with monoclonal antibodies Omy CD83 (red) and Ssa MHII (brown). Positive cells were observed associated with later stages of infection after plasmodia were disintegrated and often with engulfed myxospores. Dual labeling of CD83 revealed most cells were CD83+/MHII+. Dual-labeled cells were observed at high densities within stage 4 lesions and associated with free myxospores or with engulfed myxospores (arrowheads).
Photo: Laura Braden (AVC/AquaBounty)

Immune detection of Kudoa thyrsites by MHII+ cells. Photomicrographs of stages of cellular response. In stages 1 and 2, MHII+ cells surround and infiltrate an infected myocyte. In stage 3, MHII+ cells are recruited to the infected myocyte and surround the plasmodia (p) while disintegrating the myocyte. In stage 4, degraded plasmodia (star) and released spores are engulfed by MHII+ cells (arrowhead).
Photo: Laura Braden (AVC/AquaBounty)
The effects of smolt size on the intensity of Kudoa thyrsites infections in Atlantic Salmon
Atlantic Salmon infected with Kudoa thyrsites do not exhibit clinical signs of disease. However, protease secretion from this parasite rapidly deteriorates affected muscle in the salmon when the fillet is processed, resulting in economic loss for the grower. Earlier research suggested that the risk of K. thyrsites was reduced when salmon were transferred to sea as larger smolts. This research project involved a more robust test of the size hypothesis by conducting trials in which the prevalence and severity of K. thyrsites infections were measured in various smolt size classes following laboratory exposures.
Smolts were graded into three size classes at their respective hatcheries and were then transferred to the Pacific Biological Station and exposed to the parasite. The study revealed a significant effect of Atlantic Salmon smolt size on the risk of Kudoa thysites infections: prevalence and intensity of the infections were greater in the large size-class smolts.
A second finding of this study was the reduced overall levels of infection in the smolts that were exposed during winter months compared to the group exposed during summer months.
In summary, contrary to previous observations, smaller smolts had lower infection prevalence and intensity. The duration of this study did not permit an examination of resolution of infection, such that the consequences of smolt size on K. thyrsites infection at harvest is still uncertain.
Date: Oct. 2015 – Jun. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Marine Harvest Canada Limited; Cermaq Canada Ltd.
Project Leader: Simon Jones (DFO)
Project Team: Chris Pearce, Ian Forster (DFO); Amy McConnell (Nova Harvest Ltd.); Wenshan Liu (Manatee Holdings Ltd.)
Collaborators: Diane Morrison (Marine Harvest Canada Ltd.); Kathleen Frisch (Cermaq Canada Ltd.)
Contact: Simon.Jones@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-2-P-01-eng.html
Genomic diversity and lytic activity of bacteriophages specific against Aeromonas salmonicida, the fish pathogen that causes furunculosis
The A. salmonicida bacterium causes furunculosis in salmonids (salmon, trout, Arctic Charr, etc.). Controlling this disease, which is very harmful to the aquaculture industry, can prove to be quite demanding and fruitless, mainly because of the logistic constraints of the vaccination and the very frequent resistance of A. salmonicida to several antibiotics. In the last ten years, we have studied the genomic diversity of A. salmonicida to better understand its virulence and antibiotic resistome. We showed that a great number of A. salmonicida isolates bear antibiotic resistant genes against all approved antibiotics in Canada for aquaculture. Finding alternative treatments to prevent and cure furunculosis is now our priority.
We are evaluating the potential of bacteriophages (viruses that infect only bacteria) against furunculosis. We have isolated bacteriophages targeting A. salmonicida and characterized their genome. In vitro experimentations showed that bacteriophages have a large lytic spectrum against many A. salmonicida isolates with different virulence traits. Moreover, the bacteriophages do not infect bacteria from other species and the development of resistance of A. salmonicida against bacteriophages is weak, making these viruses promising for aquaculture. Our next experimentations will be in in vivo environment with bacteriophage cocktails against A. salmonicida infected fish. The efficiency and the administration methods of bacteriophages will be evaluated in the course of this study.
Our work will offer a new approach to treat infections due to A. salmonicida. The development of a bacteriophage treatment against the fish pathogen will aid Canadian aquaculture productivity while enabling fish farmers to have alternative treatments to decrease the use of antibiotics in food production.
Date: May 2014 – Dec. 2022
Funded by: Natural Sciences and Engineering Research Council of Canada (NSERC)
Co-Funded by: Ressources aquatiques Québec (RAQ); MAPAQ—Innovamer Program
Project Leader: Steve Charette (U Laval/IUCPQ)
Project Team: Valerie Paquet, Marie-Ange Massicotte (U Laval)
Collaborators: Nicolas Derome, Michel Frenette, Sylvain Moineau (U Laval) ; Andrée Lafaille (UdeM), Émilie Proulx (LARSEM/U Laval)
Contact: steve.charette@bcm.ulaval.ca
Website: www.amibe.org
Diagnostic validation of reverse transcription quantitative polymerse chain reaction (RT-qPCR) assays for detection of spring viremia of carp virus (SVCV)
Spring viremia of carp virus (SVCV) is the aetiological agent of an aquatic animal disease that is listed as notifiable to the World Organization for Animal Health, and reportable to the Canadian Food Inspection Agency. In this multi-year study, two RT-qPCR tests targeting either the glycoprotein or nucleoprotein gene were designed and optimized for their analytical performance. They were then evaluated along with the virus isolation by cell culture (VI) test in a diagnostic validation study to assess their fitness as tools for detection of SVCV.
Test performance metrics of diagnostic accuracy were sensitivity (DSe) and specificity (DSp). Repeatability and reproducibility were measured to assess diagnostic precision. Estimates of test accuracy, in the absence of a gold standard reference test, were generated using latent class models. Test samples originated from domesticated koi that were either virus free or experimentally infected with the virus. Two tissues, kidney and brain, were evaluated for their relative suitability as target tissues for the RT-qPCR assays. Four laboratories in Canada participated in the precision study.
Fair to high repeatability (56 to 90%) and reproducibility (22 to 87%) were observed for the RT-qPCR tests. Accuracy estimates for the RT-qPCR tests were 97 to 100% for DSe and 77 to 97% for DSp. Poor precision was observed for the VI test (33 to 55%). Accuracy estimates for the VI test were 61 to 67% for DSe and 81 to 98% for DSp. Collectively, the results show that the SVCV RT-qPCR tests are suitable tools for surveillance, presumptive diagnosis and certification of individuals or populations as SVCV free.
The diagnostic tools developed in this project for detection of SVCV are being used by member laboratories of the National Aquatic Animal Health Laboratory System in Canada. These tests enable the country to meet its obligations as a member of the World Trade Organization by preventing the spread of pathogens of international concern to other countries and to protect Canada from those pathogens not currently found within its borders. The aim now is to publish the work in a peer-reviewed journal.
Date: Apr. 2013 – Mar. 2019
Funded by: DFO – Centre for Aquatic Animal Health Research and Diagnostic (DFO – CAAHRD)
Project Leader: Sharon Clouthier (DFO)
Project Team: Tamara Schroeder, Shaorong Li, Crystal Collette-Belliveau, Jason Allen, Melissa Lindsay, Sandra Aldous, Philip Byrne (DFO)
Collaborators: Carol McClure (AquaEpi Research); Eric Anderson (Inpendent researcher)
Contact: Sharon.Clouthier@dfo-mpo.gc.ca

Koi (Cyprinus carpio) in holding tank following challenge with SVCV.
Photo: Philip J. Byrne (DFO)
Investigating the molecular mechanisms of vaccine-induced protection in Arctic Charr after infection with Aeromonas salmonicida spp. salmonicida
With respect to salmonid aquaculture, one of the most important pathogens due to high mortality and antibiotic usage is the causative agent of furunculosis, Aeromonas salmonicida spp. salmonicida. The host response during infection with A. salmonicida is well documented in Atlantic Salmon; however, little is known about the host response in other salmonids such as Arctic Charr (Salvelinus alpinus). Additionally, there is very little information on the efficacy or mechanism of protection of currently available vaccines against furunculosis when administered to Arctic Charr. Thus, the aim for this work was to use RNA sequencing to characterize the response of naïve Arctic Charr while infected with A. salmonicida, as well as charr vaccinated with ForteMicro®, a vaccine routinely administered against furunculosis. Furthermore, we were interested in assessing the response during co-administration of ForteMicro® with Renogen®, which is often the case when bacterial kidney disease is thought to be present.
Unvaccinated and vaccinated charr were IP-injected with 106 CFUs/mL and head kidney collected at 0, 8- and 29-days post-infection. Arctic Charr were extremely susceptible to A. salmonicida, with 72% mortalities observed after 31 days; however, protection of 72–82% was observed in fish vaccinated with ForteMicro® or the combination of ForteMicro®+ Renogen®, respectively. This protection was correlated with significantly elevated serum IgM concentrations, and transcriptomic analysis revealed several patterns and pathways associated with improved survival of vaccinated fish. These included a dramatically higher basal expression of humoral immune components such as complement, acute phase, and iron hemostasis, in pre-challenged, vaccinated fish.
This research demonstrated that the administration of currently available vaccines against furunculosis are effective for protecting Arctic Charr. We provide transcriptomic evidence that protection is associated with significant induction of humoral immunity in vaccinates prior to exposure to A. salmonicida, with subsequent downregulation of these processes after infection being a key feature in survivors.
Date: Jan. 2012 – Jan. 2018
Funded by: Atlantic Canada Opportunities Agency – Atlantic Innovation Fund (ACOA – AIF)
Co-Funded by: Coastal Zone Research Institute (CZRI); Natural Sciences and Engineering Research Council of Canada (NSERC); Innovation PEI; Ocean Frontiers Institute
Project Leader: Mark Fast (AVC)
Project Team: Laura Braden, Shona Whyte, Alyson Brown, Carter VanIderstine, Corinne Letendre, David Groman, Jeff Lewis, Sara Purcell, Tiago Hori (UPEI)
Collaborators: Allison MacKinnon (Novartis Animal Health Canada Inc.); Tony Manning (RPC); Mark Braceland (CATC)
Contact: mfast@upei.ca
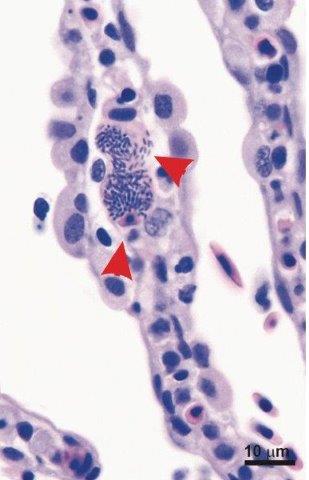
Bacteria were observed within secondary lamellae in non-vaccinated control Arctic Charr.
Photo : Laura Braden (UPEI)
Epidemiology of net pen liver disease in BC farmed salmon
Current evidence suggests that net pen liver disease (NPLD) is caused by exposure to microcystin (MC), which is a hepatotoxin produced by blue green algae. Microcystins from naturally occurring freshwater algal blooms are believed to be the major source of MC contamination in coastal waters. Historically, NPLD has been reported occasionally in wild and farmed salmonids in BC and Washington State. However, in recent years, the incidence and severity of the NPLD has increased on BC salmon farms with millions of dollars of lost production in 2014 – 2016. The increased incidence of NPLD may reflect a change in the levels of MC in BC coastal waters and raises the possibility that MC may also be impacting the health and performance of wild aquatic animals.
This project will generate knowledge that can be used to identify sites at risk of NPLD, and to improve or develop new disease management strategies. It can also be used to assess the risk of the toxin responsible for NPLD having effects on wild aquatic animals. The objectives of this project are to: 1) collect and organize various data pertaining to NPLD in BC; 2) refine methods of analysis for MC to characterize the MC-isoforms and metabolites found in salmon and other marine animals; 3) track development of NPLD using histological methods, examine presence of liver lesions in other fish, and determine the relationship with MC and/or its metabolites with disease; and 4) identify the source of MC in coastal waters.
The ability to predict whether NPLD is likely to develop would allow companies to make adjustments to their production plans that would reduce financial losses at impacted sites. This research program will also support the development of mitigation strategies, as well as identify the potential for MC to have broader ecosystem effects on wild salmonids and other animals.
Date: Apr. 2017 – Mar. 2019
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cermaq Canada Ltd.; Grieg Seafood BC Ltd.
Project Leader: Stewart Johnson (DFO)
Project Team: Pearse McCarron (NRC); Heindrich Snyman (AHC); Andrew Ross (DFO)
Collaborators: Barry Milligan (Cermaq Canada Ltd.); Tim Hewison (Grieg Seafood BC Ltd.)
Contact: Stewart.Johnson@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-P-02-eng.html
Molecular systematics of sturgeon nucleo-cytoplasmic large DNA viruses
Namao virus (NV) is a sturgeon nucleo-cytoplasmic large DNA virus (sNCLDV) that can cause a lethal disease of the integumentary system in Lake Sturgeon, Acipenser fulvescens. As a group, the sNCLDV have not been assigned to any currently recognized taxonomic family of viruses. In this study, a data set of NV DNA sequences was generated and assembled as two non-overlapping contigs. The phylogeny of NV was reconstructed using protein homologues encoded by nine nucleo-cytoplasmic virus orthologous genes (NCVOGs) identified on the contigs.
The accuracy of our phylogenetic method was evaluated using a combination of Bayesian statistical analysis and congruence analysis. Stable tree topologies were obtained with data sets differing in target molecule(s), sequence length and taxa. Congruent topologies were obtained in phylogenies constructed using individual protein data sets. The major capsid protein phylogeny inferred that ten representative sNCLDV form a monophyletic group comprised of four lineages within a polyphyletic Mimi-Phycodnaviridae group of taxa.
Overall, the analyses revealed that namao virus is a member of the Mimiviridae family with strong and consistent support for a clade containing NV and Cafeteria roenbergensis virus as sister taxa.
Since the sNCLDV group has not been assigned to any currently recognized taxonomic family of viruses, the study was designed so that the phylogeny outcomes would be taxonomically informative to the International Committee on Taxonomy of Viruses. White Sturgeon iridovirus (WSIV), a member of the sNCLDV, causes a disease that is reportable to the Canadian Food Inspection Agency in Canada. The results of this research may impact how WSIV and, by extension, other sNCLDVs are regulated by the Canadian Food Inspection Agency in Canada and by regulators of national aquatic animal health programs in Europe and the United States.
This study was published in Molecular Phylogenetics and Evolution (2018) 128:26-37.
Date: Jan. 2012 – Jan. 2018
Funded by: Manitoba Hydro
Co-Funded by: DFO - Priorities and Partnership Fund Strategic Initiative (DFO - PPSI)
Project Leader: Sharon Clouthier (DFO)
Collaborators: Rachel Breyta, Gael Kurath (USGS WFRC); Eric Anderson (Independent researcher)
Contact: Sharon.Clouthier@dfo-mpo.gc.ca
Website: https://www.sciencedirect.com/science/article/pii/S1055790318300861
Developing a novel plant derived formulation to maintain health of freshwater early life stages of fish: Efficacy and target animal safety studies
Maintenance of health in freshwater culture is critically important. Freshwater fish operations, including Atlantic Salmon hatcheries, contend with numerous pathogens, fungi and fin erosion concerns. Infections by Saprolegnia fungus alone may contribute to 10–50% egg losses. There is a need for new products that can be safely applied to all freshwater life stages to maintain health of eggs and fish in culture environments. The Huntsman egg–Saprolegnia infection model was used to evaluate efficacy of a novel plant derived formulation developed by RPS Biologiques Inc. Low concentrations of the test formulation provided comparable efficacy to the standard formalin treatment concentration against Saprolegnia declina infection of eggs (LC50 = 17.47 + 8.88%). Trials continue involving a new powder formulation.
Huntsman also completed a Target Animal Safety study based on Environment Canada’s EAF-test method, with modifications to mimic industry practices, to assess toxicity of the test formulation on Atlantic Salmon embryos, alevins and fry. Each treatment had four replicates with forty eyed eggs exposed to 50, 25, 12.5, 6.25, 3.13 or 1.56% of the test formulation along with hatchery water negative and formalin positive controls. Thirteen embryo-stage exposures and twelve fry-stage exposures were conducted with all treatments. There were no effects on mortality across treatments. Therefore, values of LC50 could not be reliably predicted. The NOEC (no observable effect concentration) for fry mortality was 50% test formulation and the LOEC (lowest observed effect concentration) was > 50%.
This project meets the urgent need to develop novel formulations that demonstrate efficacy and can be safely applied to all freshwater life stages.
Date: Jan. 2017 – Dec. 2018
Funded by: RPS Biologiques Inc.; National Research Council– Industrial Research Assistance Program (NRC–IRAP)
Co-Funded by: New Brunswick Innovation Foundation – New Brunswick Innovation Research Chair program
Project Leader: Duane Barker, Ehab Misk (HMSC)
Project Team: Anne McCarthy, Trena Hurley, Esther Keddie, Rebecca Eldridge, Ellen Fanning, Erica Harvey, Elizabeth Dowling, Dave Goodwin, Chris Bridger (HMSC); Subrata Chowdhury (RPS Biologiques Inc.)
Collaborators: Subrata Chowdhury (RPS Biologiques Inc.)
Visceral mycoses in Atlantic Salmon (Salmo salar): The role of opportunistic fungal pathogens in fish health and mortality in salmon aquaculture systems
During the summer of 2017, seven populations of Atlantic Salmon recently transferred from freshwater as smolts to net pens exhibited elevated mortality. Post-mortem examination of apparently healthy fish determined that many had extensive visceral mycoses that targeted several organs, including the spleen, liver and kidney. A subset of fish with affected organs were sampled and fungal cultures were subject to morphological and molecular analyses to identify them as dematiaceous Ascomycete fungi of the genera Exophiala and Ochroconis. These darkly pigmented, polyextremotolerant fungi inhabit a wide range of ecological niches, including animal hosts. Some are recognized as opportunistic pathogens of crustaceans, fish, birds and mammals.
This is the first confirmed report of these fungal pathogens infecting and causing significant mortality of Atlantic Salmon in BC commercial aquaculture. Although infections have been reported for both marine and freshwater fish, these fungal taxa are poorly studied as opportunistic pathogens. Their potential mode(s) of infection are unknown, and factors that influence pathogen prevalence and host susceptibility have not been described.
The objectives of the present study are to: 1) determine the temporal and spatial distribution of these pathogens in aquaculture facilities; 2) establish the prevalence of these infections among different age classes; and 3) evaluate the infection process in controlled trials to confirm isolate infectivity and their impact upon fish health. Water treatment systems and cultural practices will be evaluated to determine whether they influence the incidence of infection, with a view to developing remedial actions.
Date: May 2018 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Cermaq Canada Ltd.
Project Leader: Simon Jones (DFO)
Project Team: Paul de la Bastide, Terrie Finston, Will Hintz (UVic)
Collaborators: Danielle New (Cermaq Canada Ltd.)
Contact: Simon.Jones@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/18-p-05-eng.html
Assessing the effects of high oxygen freshwater saturation on Atlantic Salmon (Salmo salar) growth and overall health within a simulated commercial hatchery setting
Atlantic Salmon is a diadromous fish species and spends about 50% of its entire life cycle in culture conditions in freshwater land-based hatcheries. A critical aspect in commercial hatcheries is the provision of appropriate levels of oxygen within the freshwater environment to provide optimal growing conditions. Oxygen can represent a significant operational cost to the hatchery while gas bubble disease can develop in the absence of appropriate control of the total gas pressure. The new proprietary technology examined in this study yields significant advantages to freshwater facilities for Atlantic Salmon culture operations.
This project provided benchmarking between triplicate tanks receiving ambient freshwater dissolved oxygen (DO2) concentration (90% ± 10%) and triplicate tanks receiving added dissolved oxygen DO2 concentrations of either 150% ± 10% or 200% ± 10%, respectively, in simulated commercial hatchery operations starting with 3 g Atlantic Salmon fry and using preplanned density and weight triggered cutbacks until smolting. Study water was created on demand using a proprietary gas infusion system to increase measured dissolved oxygen concentrations while removing nitrogen from the water to maintain water total gas pressure at near 100%. Specific growth rate and Fulton’s condition factor were calculated using data collected either during monthly non-lethal sampling from each tank or during planned cutbacks that match the simulated production plan based on stocking density. Mortalities were recorded from each tank during the project and overall fish health assessed by lethally sub-sampling each tank population during cutbacks (n=10 × 3/group), including hematocrits and general necropsy. Preliminary data analysis indicates that fish held in 150% and 200% DO2 saturation had lower cumulative mortality (by 2.33% and 8.11%, respectively) and higher mean growth rate (by 47% and 44%, respectively) compared with controls held within ambient conditions. Interestingly, while fry held in 150% and 200% oxygen saturation had similar overall performance, only fish exposed to 200% oxygen saturation had significantly lower hematocrit values, which returned to ambient fish levels in less than ten days after treated fish returned to either ambient fresh or seawater conditions, with no significant mortalities from the control group.
Date: Aug. 2017 – Dec. 2018
Funded by: GIS Gas Infusion Systems Inc.
Co-Funded by: New Brunswick Innovation Foundation – New Brunswick Innovation Research Chair program
Project Leader: Ehab Misk (HMSC)
Project Team: Ellen Fanning, Anne McCarthy, Trena Hurley, Esther Keddie, Erica Harvey, Jamie Carpenter, Howard Streight, Phil Wiper, Cailey Dow, Elizabeth Dowling, Dave Goodwin, Chris Bridger (HMSC)
Collaborators: Mike Beattie (GIS Gas Infusion Systems Inc.)
Contact: ehab.misk@huntsmanmarine.ca
Website: Huntsmanmarine.ca

Study Tanks Setup. Three tanks are dedicated for each dissolved oxygen (DO2) saturation group, 100% ± 10%, 150% ± 10%, and 200% ± 10%. Water is gravity fed to study tanks from three black header tanks where the GIS Gas Infusion Systems technology is located. Water DO2 is automatically and continuously monitored to ensure study requirements remain within the acceptable ranges. Fish were fed to satiation with automatic feeders and manually. Photoperiod was adjusted biweekly based on the NRC sunrise/sunset calculator.
Photo: E. Misk (HMSC)
Fry/parr infection model to study Saprolegnia parasitica infections for broodstock related and novel treatment development applications
Saprolegnia parasitica is responsible for fungal water mould infections of freshwater fish in hatcheries. Infections are characterized by the growing of fungal mycelia on fish skin and fins that is promoted by stress, including water quality and/or handling. Saprolegniasis may be treated using several presently available options, such as formalin, iodophores, salt, or hydrogen peroxide, but each has its limitations resulting in a quest to develop further alternative therapeutants.
Huntsman advanced its Saprolegnia research capacity by adding an Atlantic Salmon fry/parr specific infection model. Several virulent pure Saprolegnia parasitica isolates have been collected and identified using ITS sequencing of its genomic DNA. Isolates are cultured on cornmeal agar (CMA) plates to obtain fungal growth then moved to Sabouraud Dextrose Broth (SDB) and later collected and washed with hatchery water to produce infective spores. Up to 100,000 healthy spores/mL have been mass produced on a plate, evaluated for viability, then used to infect fish soon after being released from the sporangium. Stress induction to enhance infection can be achieved as either transient during exposure through appropriate handling or prolonged using cortisol implants. Combinations of spore load, water flow, and stress induction have been demonstrated to either produce severe synchronous infection in a shorter duration or longer duration to address variable experimental needs. Infection outcomes can be evaluated by survival analysis and correlated with spore counts at several time points during infection.
Having access to consistent, reliable and predictable infection models is essential to evaluate efficacy or screen candidate treatments. The fish Saprolegnia parasitica will be used at the Huntsman Marine Science Centre to aid in the development of new treatment alternatives, as well as used to explore fish and family resistance to fungal infections in salmonid breeding programs.
Date: Apr. 2018 – Dec. 2018
Funded by: New Brunswick Innovation Foundation – New Brunswick Innovation Research Chair program
Project Leader: Ehab Misk (HMSC)
Project Team: Anne McCarthy, Trena Hurley, Esther Keddie, Cailey Dow, Peter Bartlett, Howard Streight, Chris Bridger (HMSC)
Contact: ehab.misk@huntsmanmarine.ca
Website: Huntsmanmarine.ca

Atlantic Salmon parr infected with Saprolegnia parasitica showing fungal growth on skin and fins.
Photo: Ehab Misk (HMSC)
Environmental interactions
Effect of aquaculture inputs and extractions on aquatic ecosystem ecology
The Aquaculture Ecosystems Interactions Program (AEIP) supports long-term, collaborative, multidisciplinary research on ecosystem-level effects of aquaculture. Research under the program theme “Effect of aquaculture inputs and extractions on ecosystem ecology” considers the complexity of environment processes that may affect valued ecosystem components as a direct or indirect consequence of changes in the abiotic and biotic environment. This program provides seed funding for program theme leaders to bring together internal and external scientific expertise needed to develop an understanding of the persistent, cumulative and far-field effects of present aquaculture activities on complex aquatic ecosystems.
Initial collaborative research under this theme is focusing on bivalve aquaculture. Previous studies have suggested that shellfish populations, depending on the circumstances, may represent a significant positive ecosystem service or may have negative trophic interactions that possibly result in cascading food web changes and alteration of ecosystem functions.
Field studies at multiple spatial and temporal scales are quantifying changes in pelagic ecosystem structure under different intensities of culture. Studies at the scale of the feeding zone around mussel socks will determine the relationship between mussel biomass, current speed and degree of phytoplankton reduction. Larger-scale phytoplankton reduction is measured using in situ and remote sensor mapping approaches. Investigations of the effect on zooplankton biomass and diversity are based on size-spectra and genetic material analysis of zooplankton collected from multiple embayments containing different intensities of mussel culture. Observed changes in pelagic community structure over different spatial and temporal-scales will be used to validate and refine bay-scale ecosystem model predictions.
Research conducted under this project addresses priority aquaculture-ecosystem questions relevant to the Department’s strategic outcomes: Sustainable Aquatic Ecosystems; and Economically Prosperous Maritime Sectors and Fisheries.
Date: Aug. 2018 – Ongoing
Funded by: DFO–Aquaculture Ecosystems Interactions Program (DFO–AEIP)
Project Leader: Peter Cranford (DFO)
Project Team: Anaïs Lacoursière, Luc Comeau, Thomas Guyondet (DFO)
Collaborators: Kim Hoang Hang (CGQ)
Contact: Peter.Cranford@dfo-mpo.gc.ca
Assessing the profitability of integrated multi-trophic aquaculture in Canada with and without a deposit-feeder component
This project compared the financial returns of Atlantic Salmon monoculture with salmon, mussel, and kelp Integrated Multi-Trophic Aquaculture (IMTA), and salmon, mussel, kelp, and green sea urchin IMTA in the Bay of Fundy. The results will help the industry to determine the economic viability of IMTA.
We used a discounted cash-flow analysis to build on previous IMTA analyses in Canada, incorporating updated and real-world financial information. We found that three-species IMTA production, particularly with price premiums on IMTA-produced salmon and mussels, can lead to higher net present values than monoculture. However, increased capital and operational complexity may deter investor interest in farm-scale IMTA operations. Adding green sea urchins as a benthic species was found to reduce IMTA farm profitability, but likely resulted from the small size of the sea urchin operation we investigated and the resulting high costs.
Date: May 2013 – Jul. 2017
Funded by: Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Network Program
Project Leader: Duncan Knowler (SFU)
Project Team: Chris Pearce (DFO); Mark Carras (SFU)
Contact: djk@sfu.ca; Chris.Pearce@dfo-mpo.gc.ca
Website: www.cimtan.ca
Evaluation of benthic far-field and area recovery effects from aquaculture within the Letang Inlet, New Brunswick
To evaluate benthic far-field enrichment associated with finfish aquaculture, sediment samples were collected in two areas 200-600 meters from facilities within the Letang Inlet, New Brunswick, Canada and one reference area (> 3000 meters). For changes and recovery, these were collected annually (2012-2015) to monitor a full three-year production cycle. These samples were used to measure macrofaunal and microbial communities, total microbial biomass, fecal coliform, sediment grain size, sediment organic content, and the concentrations of sterols, vitamin E, and heavy metals.
Measurements proximate to aquaculture areas had greater sediment metal concentrations but the annual patterns did not show increased metals during the period of active culture. There were also differences in environmental variables but again annual changes did not correlate with a cycle of active culture. Each area had a distinct macrofauna community with similar annual changes regardless of proximity to aquaculture. Microbial community structure also exhibited annual change but not correlated with active culture. Compared to the reference area, the two aquaculture areas did have higher levels of metals and organic enrichment and macrofauna showed episodic shifts between abundance and biomass dominance, but it was uncertain as to whether this was a response to aquaculture or a change typical of the sampling areas.
Combined, these findings indicated that coastal-scale environmental factors were likely responsible for driving the largest changes in the benthic environment, irrespective of adjacent fish farm activities, and that the current 3-year production cycle produced few, if any, detectable short-term far-field effects.
Date: Jun. 2013 – Jun. 2016
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Northern Harvest Sea Farms Ltd.
Project Leader: Andrew Cooper (DFO); Gerhard Pohle (HMSC)
Project Team: Rebecca Milne, Lou Van Guelpen (HMSC); Marc Blanchard (DFO); Robert Findlay (U Alabama); Robert Clarke (PML); Karl Whelan (Eastern Charlotte Waterways Inc.)
Collaborators: Larry Ingalls (Northern Harvest Sea Farms Ltd.)
Contact: Andrew.Cooper@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/M-13-01-003-eng.html
Effect of wind forcing on the oceanographic conditions of Fortune Bay–Belle Bay: Identification of changes in water physical conditions and ocean currents, and development of a forecasting tool
The understanding and accurate reproduction of ocean stratification and currents is key to assess aquaculture-environment interactions and to mitigate potential issues such as fish pathogen, pest and organic matter dispersion and cumulative effects.
Results of recent research have indicated that in most of Belle Bay (the inner part of Fortune Bay, Newfoundland and Labrador), tides account for less than 5% of the ocean current variability. Wind forcing, a non-deterministic force (as opposed to tides) and water physical structure (i.e., stratification) have, on the other hand, a significant influence and result in complex ocean dynamics.
This project aimed to identify and describe some of the dominant mechanisms responsible for short-term upwelling and downwelling events, and associated surface and sub-surface oceanic circulation induced by wind forcing in Fortune Bay. To this end, a comprehensive observation program was executed using oceanographic moorings, allowing simultaneous measurement of water column stratification and currents over the course of a year or more at multiple sites. Along with the observations, a numerical model is being implemented to help understand the processes involved, to get a spatial view of the ocean circulation, and to predict the transport of variables of interest (e.g., virus, sea-lice or organic material originating from aquaculture farms).
Results from this project will inform the aquaculture industry as they conduct their operations, as well as inform the regulators in matters such as fish pest and pathogen management, release of organic matter into the environment, site selection and ecosystem management.
Date: Apr. 2015 – Jun. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Newfoundland Aquaculture Industry Association (NAIA); Cold Ocean Salmon Inc.; Northern Harvest Sea Farms Ltd.; French Research Institute for Exploration of the Sea (IFREMER)
Project Leader: Sebastien Donnet, Andry Ratsimandresy (DFO)
Project Team: Dwight Drover, Pierre Goulet, Shannon Cross, Guoqi Han (DFO); Pascal Lazure (IFREMER)
Collaborators: Mark Lane (NAIA); Julia Bungay (Cold Ocean Salmon Inc.); Jennifer Caines (Northern Harvest Sea Farms Ltd.)
Contact: Sebastien.Donnet@dfo-mpo.gc.ca; Andry.Ratsimandresy@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-N-02-eng.html
Novel sensors for fish health and welfare
The coastal ocean is a challenging environment for aquaculture. Beyond obvious weather-related risks to infrastructure and worker safety, there are biological risks to cultured fish in two main areas: (1) oceanographic and climate challenges to fish physiology and welfare, and (2) presence of pathogens, harmful microalgae and other organisms that affect fish health. Working within the Ocean Frontier Institute with major Nova Scotia ocean technology companies, Vemco Amirix and Realtime Aquaculture, and New Brunswick-based Cooke Aquaculture, we will combine novel sensors to bring new standards to in-water sensing applied to the welfare and management of farmed fish.
Oceanographic sensors provide a framework to understand the farm environment, and forecast/model conditions as they relate to both fish behaviour, as well as the pathogen field in ambient waters. Taken together, these tools and data analysis present cutting-edge improvements in the sustainability of fish farming and will allow us to:
- Provide real-time data on oxygen and temperature from multiple cages at fish farming sites, using wireless, networked systems.
- Develop software delivery systems for cage sensors, specific to farm management needs.
- Develop protocols for data assimilation and modelling to enhance forecasting of oxygen/temperature data.
- Establish an array of fish acoustic tags and cage sensors to quantify 3D fish and cage position, and its relationship to environmental conditions.
- Determine relationships between oceanographic conditions, pathogen occurrence, and fish response/behaviour.
- Produce metagenomic profiles of pathogens, pests, and harmful algae at fish farms and surrounding waters.
The real-time monitoring and prediction of environmental drivers of stress in cultured salmon using novel sensors will be used as an early warning system that will improve management practices and, concomitantly, fish welfare.
Date: Apr. 2017 – Mar. 2020
Funded by: Ocean Frontier Institute
Project Leader: Jon Grant (Dalhousie U)
Project Team: Julie Laroche, Jinyu Sheng, Ramón Filgueira (Dalhousie U)
Collaborators: Cooke Aquaculture; Real Time Aquaculture; VEMCO Amirix Systems Inc; GAIN Horizon 2020
Contact: Jon.Grant@dal.ca
Website: https://www.ofi.ca/research-projects/novel-sensors-for-fish-health-welfare
The role of filter-feeders in coastal ecosystem functioning
This research program will generate fundamental scientific knowledge that can be directly applied to improving coastal zone management in Canada, and will help to promote sustainable aquaculture practices worldwide.
Human interactions with the coastal zones of our oceans can compromise marine ecosystem health. For example, the cultivation of bivalves can alter the food supplies available for other species. In addition, the man-made structures that are deployed in the ocean for this purpose constitute new habitat that can be colonized by other species such as tunicates. Assessing the overall effects of bivalve aquaculture on the ecosystem relies on our understanding of the feeding behaviour of bivalves and tunicates. These so-called filter-feeders feed by straining small food particles from water, typically by passing the water over a specialized filtering structure. Despite the importance of filter-feeders in coastal ecosystems, some critical aspects of their feeding behaviour are still not clear.
This proposed research program will improve our understanding of the feeding behaviour of bivalves and tunicates. This goal will be achieved by performing experiments in the field and under controlled conditions in the laboratory, for example, to explore the effect of a higher future ocean temperature on filter-feeder feeding rates. The outcomes of these experiments will be used to improve existing mathematical models that could be used to predict the behaviour of these species under current and future ecosystem scenarios such as climate change or human modifications to the coastal zone. Using these improved models, we will be able to explore the effects of mussel and oyster aquaculture on the environment and consequently provide recommendations to guarantee the sustainability of this important economic activity.
Date: Apr. 2017 – Mar. 2022
Funded by: Natural Sciences and Engineering Research Council (NSERC) – Discovery Grant
Project Leader: Ramón Filgueira (Dalhousie U)
Contact: Ramon.Filgueira@dal.ca
Website: www.ramonf.com
Robustness of alternative benthic impact indicators at aquaculture sites across different farm and environmental conditions, and quantification of relationships with benthic communities for proxy use
There is a recognized need for an expanded range of performance indicators that are of use in managing effects from biochemical oxygen demanding (BOD) matter effluents in soft-bottom environments. Previous research (2014-16) developed alternative methods that accurately and precisely quantify total free sulfides (TFS) and dissolved oxygen (DO) in sediment pore-water. Initial work under this project (2016-19) has been to quantify the spatial and temporal variability in these new methods, and test them under a range of aquaculture and environmental conditions in Canada to further assess their robustness and applicability for detecting BOD matter impacts.
In 2018, project objectives have been expanded to include determination of the empirical relationships with benthic community biodiversity as the scientific basis of proxy use as a management tool. This expansion is in the interest of the future provision of complete science advice on the general application of these new methods in the regulatory context. Measurements of TFS and DO made with the new methods have also been accompanied by organic content, sediment grain size, water content, and the traditional method for measuring TFS in attempts to develop a multi-indicator approach.
Results from this project will help inform aquaculture decision-makers of additional tools that can be used to increase confidence in regulatory decisions with respect to the management of benthic impact from organic enrichment at aquaculture sites.
Date: Apr. 2016 – Mar. 2020
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Lindsay Brager (DFO)
Project Team: Peter Cranford, Brent Law, David Wong, Fred Page, Chris McKindsey, Andréa Weise (DFO)
Collaborators: DFO - Aquaculture Monitoring and Modelling Program (DFO - AMMP); Pacific Aquaculture Management Division (DFO)
Contact: Lindsay.Brager@dfo-mpo.gc.ca
Website: http://dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-M-08-eng.html

Sediment sieving for the collection of infauna.
Photo: Peter Cranford (DFO)

Pore-water extraction and dissolved oxygen measurements being taken from sediment cores.
Photo: Peter Cranford (DFO)

Part of the field set-up for measurement of total free sulfides, shown here during sampling at a shellfish site.
Photo: Lindsay Brager (DFO)
Developing hard-bottom indicators from BC archived benthic video surveys associated with aquaculture activities: Rock-cliff sponge community
A significant knowledge gap exists regarding the environmental effects of finfish aquaculture on true hard-substrate habitats, largely due to logistical hindrances associated with sampling remote rocky steep habitats.
Benthic ROV video surveys were carried out at a rocky cliff setting in British Columbia to characterize epifaunal communities and substrate composition. Epifauna were dominated by glass sponges, squat lobsters, plumose anemones and shrimp, while the substrate was comprised of rock wall, skeletal sponge matrix, and cobble. Taxa-specific responses were observed in relation to modelled depositional carbon flux. For example, sulfide-oxidizing bacteria varied in abundance up to a modelled depositional carbon flux of ~2 g C m-2 day-1 where it sustained a 50% areal coverage. Although plumose anemones and shrimp showed a low frequency of occurrence at reference sites, they were sometimes abundant at a higher carbon flux within the near-field zone of the aquaculture sites. Glass sponges revealed an inverse relationship with aquaculture waste outputs at a lower carbon flux.
In the absence of a chemical surrogate for benthic organic enrichment, future research should focus on: 1) the response of various taxa to depositional gradients; and 2) the potential role of taxa as secondary indicators of aquaculture activities associated with rock-cliff communities.
Date: Sep. 2011 – Mar. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Co-Funded by: BC Government; Ocean Dynamics Canada
Project Leader: Terri Sutherland (DFO)
Project Team: Andrea Sterling, Michelle Ou (DFO)
Collaborators: Bernie Taekema, Kerra Shaw (DFO)
Contact: Terri.Sutherland@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2011-P-13-eng.html

Sponge and squat lobster community on rock cliff setting.
Photo: Terri Sutherland (DFO)
Metagenomics based ecosystem biomonitoring (ecobiomics)
This project aims to develop new methodologies to quantify the health of aquatic ecosystems. The methods could eventually be used to study the impact of aquaculture farms on the surrounding environment.
An important collaboration between Canadian federal government organisations (namely, DFO and ECCC, AAFC, CFIA, CFS, NRC, NRCAN, and PHAC) in the field of genomics is undertaking a large biodiversity-based project. Biodiversity is paramount in water and soil for sustaining diverse ecosystem services and economic activities across Canada. Land use disturbances are having enormous adverse impacts on the biodiversity and integrity of natural and managed ecosystems. For example, we are starting to better appreciate the enormous biological diversity found in invertebrate communities in rivers. Existing tools (e.g., microscope) have significant limitations, and allow only the “tip” of the biodiversity iceberg to be seen.
This project proposes to use significant advances in high-throughput sequencing and DNA barcoding to develop cost-effective metabarcoding applications. The invertebrate “zoobiome” of rivers in Atlantic Canada is examined, and contrasted with the surrounding land usage and habitat use by fish such as salmonids. This new approach to salmonid habitat assessment will be combined with salmon gut content analysis using non-lethal sampling and the same metabarcoding approach. This innovative work will examine food web connections in an unprecedented informed manner, revealing salmonids feeding habits and how they use their habitat.
This work will provide indicators of ecosystem health related to invertebrate biodiversity. We will quantify the impact from nutrients / eutrophication and land disturbance, and develop cumulative effects models, linked to key policy objectives, supporting responsible resource development.
Date: Apr. 2016 – Mar. 2021
Funded by: DFO - Genomic Research and Development Initiative (DFO-GRDI)
Project Leader: Nellie Gagné (DFO)
Project Team: Royce Steeves, Mélanie Roy (DFO); Donald Baird, Alex Bush (UNB); Vincent Mercier (ECCC)
Contact: Nellie.Gagne@dfo-mpo.gc.ca

Collection of benthic samples in New Brunswick rivers.
Photo: Parks Canada

Homogenisation of benthic samples prior to DNA extraction and metabarcoding.
Photo: Nellie Gagné (DFO)
Development and validation of a biomonitoring tool to assess the impacts of salmon aquaculture on marine benthic communities using metabarcoding
Assessments of benthic environmental impact of salmon aquaculture have generally focused on changes in macro-invertebrate communities based on morpho-taxonomic identification, or have relied heavily on abiotic proxies (e.g., sulfides), depending on local and national regulatory standards. Morpho-taxonomy can provide meaningful assessment of sediments, but is time-consuming and expensive. Abiotic proxies can provide rapid estimates of organic enrichment status; however, the accuracy of these estimates are confounded by difficulties generalizing a simple relationship between benthic fauna and geochemistry, largely due to site-specific responses.
Environmental DNA (eDNA) metabarcoding is a new method to characterize species diversity and can substantially contribute to benthic impact assessments of fish farms. This research substantiates the potential of eDNA metabarcoding for augmenting benthic monitoring approaches for salmon farms in Western Canada by rapidly providing more cost-effective and accurate biological measures than morpho-taxonomy methods.
In this study, we collected sediment samples at six salmon farms with varying sediment types in British Columbia. To represent more traditional approaches to benthic monitoring, we measured a variety of environmental variables known to be related to organic enrichment and collected both traditional morph-taxonomy and classic DNA barcoding data on benthic polychaetes. We supplemented this with eDNA metabarcoding data characterizing foraminifera and metazoa composition in the same sediment samples to enable a comparison of results among methods.
Thus far, we have found that foraminifera alpha diversity generally increases with increasing distance from fish cage edge, and that farm site explained more variation in community composition than other environmental variables. Overall, we determined that foraminifera diversity and composition estimates, as characterized by eDNA metabarcoding, generated signals consistent with benthic biodiversity being impacted by salmon farming despite that farm-specific responses were observed in different analyses.
Start Date: Apr. 2016 – Mar. 2019
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Cathryn Abbott (DFO)
Project Team: Xiaoping He, Terri Sutherland, Scott Gilmore, Kristi Miller-Saunders, Kara Aschenbrenner, Kerra Shaw (DFO)
Collaborators: Jan Pawlowski (U Geneva)
Contact: Cathryn.Abbott@dfo-mpo.gc.ca
Website: http://dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-P-06-eng.html

Macrofauna being extracted from benthic mud using running water and a sieve.
Photo: Scott Gilmore (DFO)

The polychaete Sternaspis affinis on the collecting sieve.
Photo: Scott Gilmore (DFO)
Developing the benthic component of IMTA to reduce the impact of organic nutrients from fish farms and evolving standard operating procedures
Integrated Multi-Trophic Aquaculture (IMTA) is an advanced ecological engineering technique that has been developing in Canada. IMTA mimics a natural ecosystem by combining the farming of multiple, complementary species from different levels of the food chain in a way that allows the uneaten feed, wastes, nutrients and by-products of one species to be recaptured and converted into fertilizer, feed and energy for the growth of the other species. The goal of this project was to develop the benthic portion of the IMTA system for open water marine aquaculture sites.
This research demonstrated that it is possible to hatch and raise sea cucumbers, sea urchins and scallops for use in IMTA operations in potentially large numbers. It was determined that the water supply needs to be ultra-filtered to remove copepod predators, which can destroy the three target species’ larval year classes in a matter of days. Hatchery-reared sea urchins showed very high growth rates and reached commercial size within 24 months. They assimilated approximately 70% of the food provided, which was at a level similar to the small food particles that would be available from a salmon farm. Baseline bacterial screening on cultures showed a large variety of bacterial species, the vast majority that were non-pathogenic.
The project showed that the evaluated species could potentially be used to assimilate some of the farm nutrients, resulting in less organic deposition on the sea bottom, and bringing the IMTA concept of biologically remedying aquaculture waste one step closer to being commercially viable.
Date: Apr. 2014 – Mar. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-funded by: Kelly Cove Salmon Ltd.
Project Leader: Shawn Robinson (DFO)
Project Team: Terralynn Lander, Craig Smith (DFO)
Collaborators: Keng Pee Ang (Kelly Cove Salmon Ltd.)
Contact: Shawn.Robinson@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/M-14-01-002-eng.html

Sea cucumbers feeding on salmon waste nutrient particles in an IMTA experimental tank.
Photo: Terralynn Lander (DFO)
A growing interest in seaweeds – Applied research and community engagement to support commercialization opportunities in British Columbia
Despite a clear market demand for a broad range of seaweed products (food, alginates, bioethanol, antiviral agents, fertilizers, etc.), and a coastal environment that supports one of the greatest diversity of species in the world, very little movement has been realized with respect to the development of a seaweed aquaculture sector in western Canada. Why?
Integrated Multi-Trophic Aquaculture (IMTA) research has delimited the spatial extent of the wastes generated from fish farm operations, revealing that the use of extractive species such as seaweeds offers an opportunity to capitalize on these inorganic wastes and could generate a substantial revenue stream given the high demand and market value of such products. Furthermore, the inorganic fraction has a much broader spatial impact downstream of a fish farm, and these wastes represent a clear commercial opportunity for co-culture while offering important ecosystem services.
Over the past five years, we have demonstrated the feasibility of kelp-salmon and kelp-shellfish co-culture at commercial aquaculture sites around Vancouver Island, British Columbia. Kelp aquaculture is an economic opportunity that is well aligned with the sustainability and environmental stewardship values of coastal First Nations and of many Canadians. Presently, we have established partnerships with several First Nations to build capacity for kelp commercialization in their communities, throughout coastal British Columbia. To this end, kelp seed (Saccharina latissima, with plans to later include Alaria marginata and Nereocystis luetkeana) is being planted in the winter of 2018/2019 with a focus on training members of the partner First Nations and North Island College students in kelp aquaculture techniques.
Date: Oct. 2015 – Sep. 2018
Funded by: Natural Sciences and Engineering Research Council of Canada – Industrial Research Chairs for Colleges Grant (NSERC-IRCC)
Co-funded by: Natural Sciences and Engineering Research Council of Canada (NSERC) – Engage Grant
Project Leader: Stephen Cross (NIC)
Project Team: Allison Byrne (NIC)
Collaborators:: Cermaq Canada Ltd.; Creative Salmon Company Ltd.; Grieg Seafood BC Ltd.; Mac’s Oysters Ltd.; CARTI; Odyssey Shellfish Ltd.
Contact: allison.byrne@nic.bc.ca
Website: www.nic.bc.ca/research

North Island College student research assistant measures sugar kelp (Saccharina latissima) at a salmon farm in the Broughton Archipelago, British Columbia.
Photo: North Island College
The development of video monitoring methods (camera orientation) and management thresholds to characterize fish farm impacts on hard-bottom substrates
Visual-monitoring tools have been recommended for hard-bottom substrates since conventional sampling techniques (e.g., grab and core samplers) are restricted to soft-bottom substrates. Common visual-monitoring tools consist of ROV-video forward-facing (FF) surveys or downward-facing (DF) video-drift surveys.
One objective of this project was to compare epifaunal abundance derived from tandem FF and DF video orientations along with continuous benthic surveys at marine finfish aquaculture sites. Special attention was given to mat-forming taxa (e.g., sulfide-oxidising bacteria, opportunistic polychaete complex (OPC)) associated with management thresholds in the existing aquaculture activities regulation. In terms of areal coverage estimates (%) of sulfide-oxidising bacteria, strong relationships were observed between FF and DF video orientations across all substrate types. The strongest FF vs DF relationship in this study occurred with OPC, which was limited to a single mixed substrate site. Epifaunal abundance (count data) revealed relatively strong relationships between camera orientations for fine and mixed substrates relative to that of vertical rock wall settings. Similar observations occurred for estimates of both sessile (dominated by plumose anemones) and motile taxa guilds. Weaker relationships associated with the rock wall substrate are likely due to alternating blackout periods experienced by each camera orientation as the ROV passes by vertical or ledge habitats.
In general, the use of tandem FF and DF video orientations may provide robust abundance estimates for : 1) environmental settings made up of a variety of substrate grades, and 2) structural or mat-forming taxa.
Date: Sep. 2010 – Mar. 2017
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Terri Sutherland (DFO)
Project Team: Andrea Sterling, Mike Bradford (DFO)
Collaborators: Kerra Shaw, Nathan Blasco (DFO)
Contact: Terri.Sutherland@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2010-P-10-eng.html
Structure and function of the salmon farm reef
This project sought to document the “reef” communities at finfish farms in British Columbia to illustrate the positive aspects of this bio-physical habitat as well as the potential risks and management implications of the communities present.
The presence of fish farm infrastructure provides habitat for native marine flora and fauna from the surrounding environment, in many ways acting as an artificial “reef”. In this study, the biomass and species composition of biofouling was monitored seasonally at three finfish farms in British Columbia.
Biomass on the billets ranged from 0.2—21 kg/m2. Broadly, two types of communities were observed: mussel-dominated with low biodiversity (in areas with freshwater influence), and diverse, i.e., >50 species (in highly mixed areas). The filter-feeding capacity of mussel-dominated communities was estimated at 15 million litres per day, from a total farm community weighing approximately 20 MT. Hard plastic plates were deployed at each farm, at 1 m depth, and analyzed by a taxonomist at the end of the project; over 70 species were identified from 12 phyla.
An educational poster was commissioned to visualize the biofouling community results and key nutrient pathways at a salmon farm.
Date: Apr. 2016 – Sep. 2018
Funded by: British Columbia Salmon Farmers Association – Marine Environmental Research Program (BCSFA – MERP)
Co-funded by: Cermaq Canada Ltd.; Creative Salmon Company Ltd.; Grieg Seafood BC Ltd.; Marine Harvest Canada
Project Leader: Stephen Cross (NIC)
Project Team: Allison Byrne (NIC), Chris McKindsey (DFO)
Collaborators: Cermaq Canada Ltd.; Creative Salmon Company Ltd.; DFO; Grieg Seafood BC Ltd.; NIC – CARTI; SEA Vision Group Inc.
Contact: allison.byrne@nic.bc.ca
Website: www.nic.bc.ca/research; https://www.nic.bc.ca/pdf/salmon-reef-poster.pdf

Macroalgae dominate the biofouling community on this experimental plate at a salmon farm off the west coast of Vancouver Island.
Photo: North Island College (NIC)
Kelp production at a shellfish hatchery to improve water quality
There is an immediate need for innovative strategies to help mitigate ocean acidification at shellfish hatcheries, in the face of changing and often unpredictable ocean conditions. Using kelp as a biological “buffer” to improve water quality at shellfish hatcheries is an environmentally-responsible means of tackling, at a small-scale, the consequences of ocean acidification.
This project tested the feasibility of sugar kelp (Saccharina latissima) culture in land-based tanks at a commercial shellfish hatchery. The overall goal was to demonstrate how kelp could be used to improve water quality for the hatchery, specifically parameters related to ocean acidification.
Kelp grew most successfully in areas of the tanks with the highest water flow, such as near air stones and in-flow valves. Compared to kelp grown in the open ocean, the biomass yield was smaller and the growing season was shorter in the tanks. Key challenges to land-based kelp culture were identified including: generating high flow to keep water temperature down and improve kelp growth/quality, and removing biofilm that formed at the water surface in the late spring when the air temperature and light availability were highest.
A five-day trial examining various water quality parameters in kelp and control tanks is planned for early 2019. Ocean acidification parameters (dissolved carbon dioxide and aragonite saturation), inorganic nutrients (total inorganic nitrogen and total inorganic phosphorus) and other seawater parameters (temperature, salinity, pH, dissolved oxygen, and light intensity) will be measured throughout the experiment.
Date: Apr. 2018 – Oct. 2018
Funded by: Natural Sciences and Engineering Research Council of Canada (NSERC) – Engage Grant
Project Leader: Stephen Cross (NIC)
Project Team: Allison Byrne (NIC)
Collaborators: Manatee Holdings Ltd.; NIC – CARTI
Contact: allison.byrne@nic.bc.ca
Website: www.nic.bc.ca/research

Dr. Stephen Cross holds up a line of sugar kelp (Saccharina latissima) at Manatee Holdings Ltd.
Photo: North Island College
Transforming the only non-salmon aquaculture site in the Bay of Fundy into an invertebrates/seaweeds integrated multi-trophic aquaculture site
Magellan Aqua Farms Inc. (MAFI) is the only non-salmon aquaculture site in the Bay of Fundy. It has been cultivating sea scallops since 2003, using principles of IMTA to control fouling by using the green sea urchin. This similar interest in multidimensional farming brought Dr. Chopin (Chopin Coastal Health Solutions Inc.; CCHSI) and Dr. Backman (MAFI) together, in the fall of 2015, to examine ways to integrate seaweeds into a shellfish operation in an effort to diversify, environmentally and economically. The resulting collaboration included the modification of an aquaculture site for economic diversification and in anticipation of the impacts of climate change (increased temperatures and coastal acidification).
During the 2015-2016 season, they grew a few lines of two kelp species, Saccharina latissima and Alaria esculenta, to see if the site of MAFI was appropriate for kelp cultivation. During the 2016-2017 season, CCHSI and MAFI identified the optimal depth for kelp cultivation at this site. Understanding the unique characteristics of each site, with respect to halocline, currents, fouling strata and light penetration, is critical for the optimization of the production. The 2017-2018 season was used to improve the deployment, harvesting and drying steps with a small production. The two bottlenecks identified are at the levels of harvesting and drying, hence a project to develop a specifically designed seaweed boat and a new drying facility, with the equipment of the first step being directly useable for the second step, as the production is being ramped up. The 2018-2019 season will see a scaling-up of the production for the two species, as different markets are being developed for each.
Date: Sep. 2015 – Ongoing
Funded by: Magellan Aqua Farm Inc. (MAFI); Chopin Coastal Health Solutions Inc. (CCHSI)
Project Leader: Thierry Chopin (CCHSI); Steven Backman (MAFI)
Contact: tchopin@unbsj.ca; steve.backman@skretting.com

Steve Backman (left; owner of Magellan Aqua Farms Inc.) and Thierry Chopin (right; owner of Chopin Coastal Health Solutions Inc.) with their sea scallop and seaweed crops cultivated in the Bay of Fundy, New Brunswick.
Photo: Taylor Widrig (Mermaid Fare Inc.)

Underwater line of cultivated sugar kelp (Saccharina latissima) at the site of Magellan Aqua Farms Inc. in the Bay of Fundy, New Brunswick.
Photo: Steve Backman (Magellan Aqua Farms Inc.)
Can hard clams (Mercenaria mercenaria) increase the rate of eelgrass (Zostera marina) recovery in areas impacted by oyster aquaculture?
Seagrasses such as eelgrass (Zostera marina) have beneficial effects for many invertebrate and fish species, but eelgrass communities are declining in many areas of the world. In a previous study, shading from oyster aquaculture structures was shown to negatively affect eelgrass, with no significant signs of recovery for three growth seasons after the removal of aquaculture structures. Since bivalves have been found to stimulate eelgrass growth, this project aimed to determine whether the seeding of hard clams (Mercenaria mercenaria) could enhance eelgrass recovery in areas impacted by oyster aquaculture.
The addition of hard clams to denuded areas of an eelgrass bed impacted by oyster aquaculture was unsuccessful in accelerating eelgrass recovery. This was true at all clam densities tested in the study. The expected outcome of increased nitrogen levels (in sediment and leaf tissue) due to the addition of these clams was similarly not observed. Clams sown directly under lines of suspended oyster bags at an active aquaculture site failed to promote eelgrass growth, and eelgrass shoot density gradually declined over the course of the study.
Based on these results, the practice of using hard clams is not recommended as a measure to mitigate the impacts of the aquaculture industry on eelgrass. However, the study did show that denuded eelgrass patches naturally recovered after three years. Eelgrass biomass returned to background levels after two growth seasons, while shoot density showed full recovery after three growth seasons. Hence, impacts of oyster aquaculture on eelgrass beds are reversible in a timeframe similar to natural events such as ice scouring.
Considering the results of this study, the rating for the duration of effect criteria in the risk assessment for oyster aquaculture needs to be re-evaluated. This rating should take into account the timeframe of natural eelgrass recovery when assessing aquaculture impacts on eelgrass beds.
Date: May 2014 – Jun. 2017
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-funded by: L’Étang Ruisseau Bar Ltd.
Project Leader: Monica Boudreau (DFO)
Project Team: Claire Carver (Carver Marine Consulting)
Collaborators: André Mallet (L’Étang Ruisseau Bar Ltd.)
Contact: Monica.Boudreau@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/G-14-01-004-eng.html

Eelgrass percent coverage in different treatment plots at the oyster table aquaculture lease on week 9 (A), week 55 (B), week 112 (C) and in the natural eelgrass plot (D).
Photo: Marc André Mallet (L’Étang Ruisseau Bar Ltd.)
Conversion between bivalve aquaculture types: Consequences on hydrodynamics and food accessibility
In order to optimize the bivalve aquaculture industry yields in Atlantic Canada, farmers have been converting between culture techniques (i.e., on-bottom to off-bottoms) and species (i.e., mussels and oysters). These conversions usually come with a change in growing structure and crop location in the water column. Such changes can modify the water circulation, food availability and competition with natural bivalve populations in the vicinity of the farms. This project aims to measure the influence of growing structures used in Atlantic Canada have on hydrodynamics (i.e., drag generated by mussel longlines and oyster floating cages), and to measure the difference in food availability between oysters cultured on the bottom and in floating cages.
The information generated by this project will directly contribute to management decisions around new and existing farms, as well as help to improve the numerical modelling tools used to assess bay-scale carrying capacity.
Date: Apr. 2018 – Mar. 2020
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Thomas Guyondet, Rémi Sonier (DFO)
Project Team: Jeffrey Barrell, André Nadeau, John Davidson (DFO)
Collaborators: Kim Gill, Aaron Ramsay, Jarrod Gunn McQuillan (PEI Department of Agriculture and Fisheries); Réal Savoie (Bouctouche Bay Industries); Marie-Josée Maillet (NBDAAF)
Contact: Thomas.Guyondet@dfo-mpo.gc.ca; Remi.Sonier@dfo-mpo.gc.ca
Improving ecological models for a sustainable development of bivalve culture in eutrophic estuarine complexes
The carrying capacity of coastal systems for bivalve culture has typically been investigated using mathematical models restricted to a Nutrient-Phytoplankton-Zooplankton-Detritus-Cultured Bivalve representation. This project aims to develop a more detailed understanding of nutrient dynamics to accurately gauge the influence exerted by cultured bivalves. The addition of macroalgae, like sea lettuce, in a model may help better refine primary production, especially in eutrophic systems. The addition of wild bivalve population modules will also be coupled to an existing ecosystem carrying capacity model. Model development will be supported by field and experimental work to characterize distribution, abundance and growth for both macroalgae and wild bivalves. Model application will be performed for Malpeque Bay, PEI; however, the model will be given a generic structure allowing future applications to other coastal embayments.
Adding these new modules will increase model veracity and provide new insights into aquaculture-coastal ecosystem interactions, especially in quantifying the influence on species with commercial, recreational and aboriginal value.
Date: Apr. 2015 – Mar. 2018
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Thomas Guyondet (DFO)
Project Team: Romain Lavaud, Luc Comeau, André Nadeau, John Davidson, Jeffrey Barrell (DFO)
Collaborators: Réjean Tremblay (ISMER-UQAR); Ramón Filgueira (Dalhousie U); Cindy Crane (PEI Department of Communities, Land and Environment)
Contact: Thomas.Guyondet@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2015-G-11-eng.html
Assessing the effectiveness of power-washing to mitigate the movement of aquatic invasive species associated with shellfish aquaculture products
Aquatic Invasive Species (AIS) are not uniformly distributed in British Columbia coastal waters so it is important to understand the vectors contributing to their spread and to identify potential mitigation measures that could be used to prevent or, at least, limit it. A recently completed project by Curtis et al. (2015, DFO–CSAS) identified shellfish movements as a vector for the European green crab and other AIS in British Columbia.
Although government regulations aim to limit the movement of AIS, including European Green Crab, on shellfish by requiring the aquaculture industry to “rinse” their product before leaving the farm site, there remain several gaps related to the potential effectiveness of this control, as rinsing is not defined. This project addressed this gap by evaluating the efficacy of power-washing to remove AIS from cultured oysters, relative to taking no action (control) and the current industry practice of dipping culture gear in the water (industry control). Further, the testing of different combinations of power-washing intensity (psi) and duration (time) allowed us to identify an optimal combination that could be used by industry and clarified in the regulations that would reduce the risk of AIS movements. Since several AIS tunicates are listed for control under the AIS Regulations in the Fisheries Act, this project could also inform potential mitigation for other AIS vectors (i.e., recreational boats, commercial ships) where control options such as power-washing might be employed. AIS were never completely removed from shellfish by any treatment applied in this project, so some level of invasion risk remains for managers to consider mitigating. Further, the believed “rinsing” practice employed by most growers was not significantly different from untreated controls, indicating that this practice does little to mitigate the potential movement of AIS and that managers should consider revising the conditions of license that pertain to this.
This project was able to identify a treatment combination (pressure and time) that significantly reduced the amount of biofouling. Power-washing resulted in oyster loss (blown off culture gear) from 4 to 20% and increased harvest time; factors that would need to be considered in an economic evaluation of the process. All of these results could be applied to other regions where treating shellfish product (or gear) is desired to reduce the likelihood of inadvertently moving AIS.
Date: Apr. 2016 – Mar. 2018
Funded by: DFO–Strategic Program for Ecosystem-Based Research and Advice (DFO–SPERA)
Co-Funded by: Urchinomics; Nova Harvest Ltd.; University of Victoria
Project Leader: Thomas Therriault, Chris Pearce (DFO)
Project Team: Lyanne Curtis, Vanessa Hodes, Jocelyn Nelson, Calley Wasser, Julia Savery (DFO)
Collaborators: Barry Seeley (C.A.R.S. Ltd.)
Contact: Thomas.Therriault@dfo-mpo.gc.ca; Chris.Pearce@dfo-mpo.gc.ca

Power-washing oyster lines at the industry farm site.
Photo: Julia Savery (DFO)
Shellfish: Mussels
Serial knots and mesh hemicylinders as anti-predator devices on mussel culture ropes
Predation by sea ducks is a threat to mussel culture in many sites. However, in nature, mussel survivorship is enhanced by crevices and substrate features that reduce the access of predators to their preys. We developed and tested methods that mimic the effect of such substrate features by using loosely knotted spat collector ropes and mesh hemicylinders. The knots we used were the chain sinnet and variants of spool knitting knots. These are serial knots which can be knotted easily for spat collection and socking, and undone easily at harvest if desired. The study was conducted at an open site located in Cascapédia Bay, Quebec.
In four consecutive annual trials, we found that the spool knitting knots collected up to 6000 individuals per 30.5 cm of collector length. This amounts roughly to two and four times as much as was collected on the chain sinnet and unknotted control ropes, respectively. Subsequent survivorship was negligible on the controls and on the chain sinnet. Mussel abundance on the spool knitting treatment was acceptable after the first annual predation bout, but was unsatisfactory after the second one. We then ran a trial with mesh hemicylinders, or “duckshield”, as an additional protective device. Mussel abundance with duckshields was over an order of magnitude higher than on unprotected controls.
A knotting machine and an updated duckshield prototype are presently being developed. Our work will provide mussel growers with methods and equipment for increasing mussel spat collection and survivorship without negative impacts on sea ducks. Our methods entail minimal changes in longline suspension-culture techniques.
Date: Jul. 2017 – Oct. 2020
Funded by: R-D Mytis Ltée
Co-Funded by: Quebec Department of Agriculture, Fisheries and Food (MAPAQ); National Research Council– Industrial Research Assistance Program (NRC–IRAP); Centre for innovation support through research (CAIR–UQAR); La Ferme Maricole du Grand Large
Project Leader: Marcel Fréchette (R-D Mytis Ltée)
Project Team: Éric Bujold (La Ferme Maricole du Grand Large); Jean-Christian Méthot (CAIR-UQAR)
Contact: rdmytis@gmail.com
Assessing the effect of climate change-related summer heat wave on the condition and physiology of the cultured Blue Mussel (Mytilus edulis) and monitoring of the carbonate system within Prince Edward Island bays
Climate change is an issue that will impact the shellfish aquaculture industry in Atlantic Canada. There are a number of questions related to the impact of the physical effects of climate change on the environment (such as ocean acidification) in which the shellfish aquaculture industry operates, and consequently the health and health management of farmed animals.
This project quantified physiological stress (shellfish health impact) associated with increased water temperature for extended periods of time in Blue Mussel. Seeds and one-year-old mussels from six sites in PEI were subjected to heat waves of varying durations and their survival, physiological condition and feeding behaviour were measured. To assess the potential tolerance threshold to ocean acidification, parameters of the carbonate system were measured within the shellfish aquaculture environment in order to calculate current levels of saturation.
Results from this study indicate that increases in the length and intensity of summer heat waves, as a result of climate change, will likely negatively affect the condition, physiology and survival rate of the Blue Mussel. Factors such as food quantity/quality and dissolved oxygen levels, among others, will influence the extent to which the Blue Mussel is affected.
Start Date: May 2015 – Mar. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Prince Edward Island Aquaculture Alliance (PEIAA)
Project Leader: Rémi Sonier (DFO)
Project Team: Luc Comeau, Kumiko Azetsu-Scott (DFO); Aaron Ramsay (DFARD); Sarah Stewart-Clark (Dalhousie U)
Collaborators: Peter Warris (PEIAA)
Contact: Remi.Sonier@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-M-01-eng.html

Mussels.
Photo: DFO, Gulf Region
Performance of triploid mussels in Prince Edward Island
As part of mussel aquaculture, up to 75% of the mussels socked are lost throughout the grow-out phase. This has a negative effect on the ecological — and production — carrying capacity and is not an efficient use of the natural resources available to the mussel aquaculture industry, particularly phytoplankton, which is essential to enhance the ecological and production carrying capacity of an estuary or bay.
Building on a previous Program for Aquaculture Regulatory Research (PARR) project assessing mussel stock structure and density in long-line culture, this project will study the efficient use of natural resources (phytoplankton) to grow mussels, by determining the byssus (i.e., silky fibers made of proteins by the mussels that attaches to other substrates) attachment strength in wild and hatchery produced diploid and triploid mussels. Sexually-mature mussels will be collected from the field in spring and transferred to a local hatchery. Diploid and triploid larvae will be produced, grown in the field and transferred into socks. During the 1-year grow-out phase, the project’s monitoring will focus on the environment, byssus strength, feeding efficiency, and triploid composition.
The overall objective of this project is to improve the ecological footprint of the mussel leases by reducing mussel fall off and increasing mussel absorption rates, as well as to test if triploid mussels result in increased byssus attachment strength, thus increasing the likelihood of mussels remaining on the sock until harvest.
Date: Mar. 2017 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Confederation Cove Mussels Co. Ltd.
Project Leader: John Davidson (DFO)
Project Team: Luc Comeau (DFO); Réjean Tremblay (ISMER); André L. Mallet (L’Étang Ruisseau Bar Ltd.); Jose M.F. Babarro (Instituto de Investigaciones Marinas–CSIC); Aaron Ramsay (DFARD)
Collaborators: Terry Ennis, Dana Drummond (Confederation Cove Mussels Co. Ltd.)
Contact: John.Davidson2@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-G-03-eng.html
Developing diagnostic markers to assess mussel population health in response to environmental stress
One of the major challenges of the Blue Mussel aquaculture industry is identification of stressors in the aquatic environment. As climate change continues to cause changes to the aquatic environment, stressful conditions could become more prevalent. The goal of this project is to identify genetic markers correlated to stressors such as poor food availability, hypoxia, salinity, thermal, pH, and tunicate (Ciona) recruitment/removal. These markers can then be used to investigate the causes of stress within underperforming mussel populations, and to develop mitigating strategies to minimize the impacts of the underlying environmental/mechanical stressors on the long-term viability of the mussel aquaculture industry in PEI.
Stress challenges will be completed for each of the stressors of interest. Stress response will be measured using heartbeat and lysosomal destabilization for each challenge. After each challenge, mussel samples will be collected and RNA-seq analysis will be completed. Transcriptome data from RNA-seq will be analyzed to discover molecular markers related to a specific stressor. RT-qPCR will be used in this project to validate stress markers, and to evaluate stress responses.
This project is focused on generating tools to enable industry to determine and analyse the stressors that impact cultured Blue Mussels, and investigate the effects these can have on their condition and health. This project aims to be proactive in managing health issues that may arise for cultured shellfish species in relation to climate change (i.e., changes in water temperature and pH), tunicate treatment (high pressure water spray and hydrated lime) and other environmental stressors (food availability, hypoxia and salinity).
Date: Oct. 2016 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Prince Edward Island Aquaculture Alliance (PEIAA)
Project Leader: Denise Méthé (DFO)
Project Team: Sarah Stewart Clark, Stephanie Hall (Dalhousie U); Carla Hicks (DFO); Fraser Clark (Mount Allison U)
Contact: Denise.methe@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-G-04-eng.html

Left to right: Sarah Stewart-Clark (Dalhousie U), Denise Méthé (DFO), and Stephanie Hall (Dalhousie U).
Photo: Scott Jeffrey (Dalhousie U)
An analysis of the perceived impact of mussel aquaculture on catch per unit effort (CPUE) for the seasonal lobster fishery on the northeast coast of Newfoundland
Over the past two decades lobster harvesters have noted an apparent decline in lobster catch rates on the northeast coast of Newfoundland (Lobster Fishing Areas (LFAs) 4A and 4B). This has been particularly evident in the western section of Notre Dame Bay and Green Bay where mussel aquaculture is prevalent. This analysis project will use a combination of recent and historical data for lobster annual catch statistics to determine whether there is a difference in lobster CPUE (catch per unit effort) in bays with active mussel aquaculture leases as compared to those without. This will be the first comprehensive analysis of the interaction between lobster abundance and mussel aquaculture in the region.
This project provides the foundation for further investigation into the potential impact of mussel aquaculture on the commercial lobster fishery situated on the Northeast coast of NL.
Date: Apr. 2017 – Mar. 2018
Co-Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Harry Murray (DFO); Elizabeth Coughlan (DFO)
Contact: Harry.Murray@dfo-mpo.gc.ca; Elizabeth.Coughlan@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-nl-07-eng.html
A study of the reproductive patterns of the Blue Mussel (Mytilus edulis) grown in deep and shallow water sites in Notre Dame Bay, Newfoundland
Condition, growth and reproductive indices of Blue Mussel (Mytilus edulis) grown in deep and shallow sites were studied from May to December 2016 in the South Arm region of Notre Dame Bay, Newfoundland and Labrador. The region was shown to be characterized by a seasonal thermocline that formed annually in April and lasted until October, and resulted in a cold water deep layer (mean annual temperature: 4.36 ± 5.1°C) and a comparatively warmer surface layer (mean annual temperature 5.81 ± 6.3°C).The deep water culture sites studied in this project extended into the cold water layer, while the shallow sites were typically in the warmer layer above.
Mussels grown in shallow sites had longer and heavier shells than those from deep water sites, likely linked to warmer surface waters. Both reproductive and physiological condition indices were high for mussels from both culture depths in May. However, physiological condition declined for both deep and shallow cultured mussels from May through to August. Mussels from deep sites, however, had a higher overall mean dry tissue weight: wet tissue weight ratio for the study period. Reproductive indices, including measurements of reproductive output, declined for shallow mussels from May through to August, but remained high for deep mussels until August when a significant spawn occurred for mussels from both depths. Additionally, mussels from deep sites were observed to maintain themselves in a pre-spawning ripe stage for a longer period than those from shallow sites.
Variation in physiological and reproductive indices for mussels from each respective culture depth were thought to be linked to differences in the environmental characteristics of the deep and shallow water layers including rate of temperature change, and potential differences in the present study adds to the current understanding of the effects of the deep water environment on mussel phytoplankton quality and energetics. The additional data provided on reproduction patterns and the relationship to meat yield production will help highlight the benefits of utilizing deep water sites leading to increased environmental sustainability for mussel culture in subarctic coastal environments like those on the North coast of Newfoundland and Labrador.
Date: Sep. 2015 – Mar. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Norlantic Processors Ltd.
Project Leader: Harry Murray (DFO)
Project Team: Sharon Kenny, Dwight Drover, Daria Gallardi (DFO)
Collaborators: Terry Mills (Norlantic Processors Ltd.)
Contact: Harry.Murray@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-N-03-eng.html
Effect of mussel culture on long-term condition of Rock Crabs and lobsters
In suspended mussel culture, mussels may fall from farm structures that, in combination with the physical farming structure added to an area, may attract a variety of predators and scavengers like American Lobster (Homarus americanus) and Rock Crab (Cancer irroratus). Little is known of how mussel farms may impact fisheries species through ecological effects, altered fisheries productivity, distribution, or catchability. In particular, it is unclear whether farms simply attract individuals to an area, or whether the abundance of food available (mussels, and aggregated prey) at farms alter stock productivity.
This project will conduct a long-term laboratory experiment to investigate the effects of various diets that simulate the food available at mussel farm sites on the ecological and physiological performance of Rock Crabs and American Lobsters. In addition, we propose to use the laboratory data to build population models that will allow for projecting trajectories of our experimental populations over several generations. The results are designed to inform managers on the interaction and effect of mussel culture on commercial fisheries.
Start Date: Apr. 2017 – Mar. 2020
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: David Drolet (DFO)
Collaborators: Éric Bujold (La Ferme Maricole du Grand Large)
Contact: David.Drolet@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-q-04-eng.html

Experimental mesocosms used to investigate effect of diet on long-term performance of lobsters.
Photo: David Drolet (DFO)

Female lobster in its mesocosm.
Photo: David Drolet (DFO)

Rock crabs in mesocosm experiment investigating the effect of diet on long-term performance of crustaceans.
Photo: David Drolet (DFO)
Effect of mussel culture on catchability of Rock Crabs and lobsters
Interactions between mussel aquaculture and the benthic community are complex. Suspended mussel culture may attract a variety of predatory and scavenging species with the number of mussels that fall from culture structures or the physical farming structures themselves. Few scientific studies have addressed how such changes may have bottom-up effects that impact fisheries species. Impacts may include not only ecological effects, but also effects on fisheries due to altered productivity, distribution, and/or catchability of target species. Indeed, it is widely perceived by fishers that lobsters and crabs congregate in aquaculture sites and may become more difficult to catch as they feed on an abundant mussel supply and become less attracted to baited traps.
This project will examine the catchability of lobsters and crabs within and around mussel culture sites to evaluate their availability to the fishery, providing scientific information to inform managers about the influence of mussel culture sites on lobster and crab distribution and their catchability for use in evaluating requests for new mussel farms.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: David Drolet, Chris Mckindsey (DFO)
Contact: David.Drolet@dfo-mpo.gc.ca ; Chris.Mckindsey@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-q-04-eng.html
Video of behaviour in experiment investigating effect of aquaculture on catchability of lobsters.
Photo: David Drolet (DFO)
Shellfish: Oysters
Optimization of oyster production parameters in a lagoon environment
The diversification of aquaculture activities is necessary to ensure the profitability of shellfish farms in the Magdalen Islands. In this context, the development of oyster culture in the lagoon environment has aroused great interest among local aquaculturists. However, the optimization of growing and ripening operations in a lagoon context is a short-term challenge for this oyster industry.
Merinov, in partnership with local producers, is conducting a project to test various cleaning scenarios in situ during a multi-year production cycle, in order to limit the impact of biofouling on oyster production and operation costs. In addition, a section on biotechnology management will also optimize the development of the oyster industry by identifying the type of structures and densities to recommend in lagoon conditions. In addition, a techno-economic model coupled with a production schedule will be developed jointly with the three companies collaborating on the project.
The results of this research project, over two years of production, will suggest methods adapted to the development of lagoon oyster culture in the Magdalen Islands, proposing techniques to reduce biofouling and to adopt methods of culture optimizing the yield and survival of oysters.
Date: Jun. 2018 – May 2020
Funded by: Quebec Ministry of Agriculture, Fisheries and Food (MAPAQ); Quebec Ministry of Economy, Science and Innovation (MESI)
Project Leader: Nicolas Toupoint (Merinov)
Project Team: Robin Bénard, Pascale Chevarie, François Gallien, Francine Aucoin, Michèle Langford, Valérie Poirier, Claude Poirier, Denis Boudreau, Yvon Chevarie, Léonard Huet-Doyle, Stéphanie Arnold (Merinov)
Collaborators: Carlo Éloquin (Grande-Entrée Aquaculture); Sylvain Vigneau (Culti-Mer); Michel Fournier (Moules de Cultures des Îles)
Contact: Nicolas.toupoint@merinov.ca
Website: https://merinov.ca/a-propos/

Oysters
Photo: Léonard Huet-Doyle (Merinov)
Cocktail oyster production in the Bras d'Or Lake, Cape Breton
In early 2000s the Bras d’Or Lake oyster industry suffered catastrophic losses in various locations from the invasive oyster parasite, Haplosporidium nelsoni (MSX). Evidence suggests that the oyster population is slow to recover and this is consistent with the impact of H. nelsoni in other locations along the U.S. Eastern Seaboard where oyster mortalities have occurred because of H. nelsoni infections. Interestingly, there are some locations in the Bras d’Or Lake where sampled oysters test positive for H. nelsoni, yet the infections do not appear to cause mortality. Previous infection rates in some of these locations have tested as high as 30%, but normally the prevalence of the parasite is closer to 10%. The exact ranges from year to year are not well known due to limited disease monitoring activity.
One of the areas in the Bras d’Or Lake that has tested positive for over a decade, but with no observed mortality, is near the community of Potlotek First Nation. Although there is little mortality from H. nelsoni on leases near this area, oysters from this location grow slower when compared to other Bras d’Or Lake locations. In an effort to move the industry forward and to enter the market as soon as possible, this project aims to grow cocktail oysters in the slower growing regions using surface oyster aquaculture techniques.
This research could provide an earlier entry point for the Potlotek First Nation using their oyster aquaculture leases that are traditionally slow growing. If successful on the experimental leases, similar practices could be employed on other leases in the region to increase productivity on leases that are similar in nature.
Date: May 2019 – Apri. 2021
Funded by: Nova Scotia Department of Fisheries and Aquaculture
Project Leader: Rod Beresford (CBU)
Project Team: Ken Oakes (CBU); Neil Ross, Ross Scinergy (Dalhousie U); Anita Basque (Potlotek First Nation); Robin Stuart (Englishtown, Cape Breton)
Contact: rod_beresford@cbu.ca

Anita Basque (Potlotek First Nation) and Rod Beresford (Cape Breton University) near the shore in Potlotek First Nation.
Photo: Corey Katz Photography
Re-establishing the Bras d'Or Lake oyster aquaculture industry: Assessing the impact of environmental conditions as a disease mitigation strategy for cultivating oysters in an MSX positive environment
In the early 2000s, the Bras d’Or Lake oyster industry suffered significant mortality from the oyster parasite, Haplosporidium nelsoni (MSX). This was the first report of H. nelsoni in Canadian waters. The presence of this parasite means that oysters from the Bras d’Or Lake cannot be transferred out, and because of Malpeque disease outside of the Bras d’Or Lake, oysters cannot be transferred in. Thus, the Bras d’Or Lake oyster industry needs to find a solution from within.
The aim of this project is to determine if environmental conditions can support the growth of oysters in the Bras d’Or Lake while limiting disease progression and oyster mortality. Approximately 20,000 oysters will be placed across 11 different locations in the Bras d’Or Lake that represent varying temperature and salinity profiles. At each location, 50% of the oysters will be at the surface and 50% on bottom. This will allow us to determine H. nelsoni activity at these sites, at the surface and on bottom simultaneously. Over the next three years, data related to oyster growth, oyster mortality, and disease prevalence and intensity will be monitored.
Temperature and salinity are known factors that influence the progression of disease and subsequent oyster mortality, therefore, detailed monitoring of temperature and salinity will be conducted during the three year project at every site. When the project is complete, the collected data will be used to identify what temperature/salinity profiles are most effective to support oyster growth and limit the impact of the parasite.
This research could provide a means to successfully grow oysters in H. nelsoni positive waters in the Bras d’Or Lake and other areas impacted by the parasite. It could also assist in the protection of existing successful oyster industries in the region not yet affected by this parasite.
Date: May 2019 – May 2022
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-Funded by: Nova Scotia Department of Fisheries and Aquaculture
Project Leader: Rod Beresford (CBU)
Project Team: Ken Oakes (CBU); Neil Ross (Ross Scinergy Inc./Dalhousie U); Robin Stuart (Englishtown, Cape Breton); Anita Basque (Potlotek First Nation); Joe Googoo (Waycobah First Nation)
Contact: rod_beresford@cbu.ca

Hailey Shchepanik sorting oysters for deployment across the Bras d’Or Lake.
Photo: Stacey Lee (CBU)

Stacey Lee sorting oysters for deployment across the Bras d’Or Lake.
Photo: Hailey Shchepanik (CBU)
Microbial impacts on shellfish aquaculture in relationship to ocean acidification
Ocean acidification, characterized by high dissolved carbon dioxide (pCO2) levels, can negatively affect shellfish development and survival. However, it is not clear whether high pCO2 levels alone are responsible for poor performance in some West Coast shellfish operations. While hatcheries have successfully controlled levels of pCO2 for larval and nursery production through the use of buffering systems, there have continued to be high variations in batch to batch survival. This suggests that other uncontrolled aspects of the seawater composition during high pCO2 events may be at play, most notably bacterial compositions.
This study identified the microbial communities associated with hatchery and nursery culture of oysters over the life of multiple culture batches. Good and poor production batches were then compared. Communities were characterized for the incoming seawater at different levels of pCO2 and, after controlling levels of pCO2, during larval and nursery productions. Community composition shifted consistently through the season. The late season was associated with an enrichment of overexpressed viral processes. The early season had much lower expression of these genes. An effect of pCO2 on microbial communities was also observed, although this effect was less than the effect of season. Although only minor differences were present between good and poor production batches, a number of candidate bacteria that were more prevalent in poor batches are now being further investigated. If one or more are shown to be pathogenic, the industry can develop mitigation strategies and biosecurity measures to reduce exposure, which could provide economic benefits by stabilizing hatchery production.
Date: Jun. 2014 – Jun. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO– ACRDP)
Co-Funded by: Island Scallops Ltd.; Fanny Bay Oysters Ltd.
Project Leader: Kristi Miller-Saunders (DFO)
Collaborators: Barb Bunting, Rob Saunders (Island Scallops Ltd.); Brian Yip (Fanny Bay Oysters Ltd.)
Contact: Kristi.Saunders@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/P-14-02-001-eng.html
Installment of anti-predator netting to improve oyster seed survival
The performance of anti-predator nets was assessed at a small, family-owned and operated shellfish farm (beach lease) on Denman Island, British Columbia. Oyster predators such as crabs, birds, and sea stars are natural and important components of the marine intertidal ecosystem. In some cases, the losses due to predators may not justify the various costs of installing anti-predator netting. In the last few years, however, predation of oyster seed by red rock crabs at this farm on Denman Island had resulted in almost total losses of their oyster seed investment, prompting the installment of anti-predator nets.
A relatively new strategy being adopted to protect oyster seed is to outline a section of beach with PVC-coated wire fencing, and place large clam nets overtop of this area. As part of this project, North Island College researchers and students helped install these nets as well as more traditional mesh oyster pouches. Crab presence and seed survival were monitored in netted and control sections of beach.
The company saw an immediate benefit from both types of netting. Crabs were eating significantly less of the oyster crop within the protected tenure spaces. An effective solution against predators must consider many factors in addition to oyster survival, such as upfront and maintenance costs, the potential for marine plastics pollution, habitat of native species, and human activity near the farm tenure.
This collaboration helped Han Pacific Shellfish Ltd. overcome a production challenge while providing four students with valuable experience working directly with shellfish growers.
Date: Jun. 2017 – Dec. 2017
Funded by: Natural Sciences and Engineering Research Council (NSERC) – Engage Grant
Project Leader: Allison Byrne (NIC)
Collaborators: Han Pacific Shellfish Ltd.; NIC – CARTI
Contact: allison.byrne@nic.bc.ca
Website: www.nic.bc.ca/research

North Island College student research assistant working at the oyster farm on Denman Island, British Columbia.
Photo: North Island College
Seasonal mortality in pacific oysters in Baynes Sound: Effect of environmental variables, spawning, and pathogens
Pacific Oyster summer mortality syndrome has occurred throughout the world since the 1970s. Globally, the phenomenon has been linked to elevated temperatures, spawning events, high nutrient levels, and pathogens. Over the last five years it has emerged in Baynes Sound, an area that produces over 50% of the Pacific Oysters in British Columbia, causing mortalities of up to 95% in 2015. The objectives of the present study are to: (1) identify environmental and physiological conditions that correlate with Pacific Oyster mortality, and (2) identify potentially pathogenic bacteria associated with mortality events.
From May to September (2017–2018), four sites (suspended and intertidal) were monitored to track oyster mortality, temperature, light, turbidity, salinity, dissolved oxygen, chlorophyll-a, pH, sediment characteristics, and the diversity of phytoplankton and zooplankton. Oyster mortality was monitored weekly and individuals were collected weekly to examine the resident bacterial populations and gamete development. In 2018, mortalities were 50% in suspended culture and 25% in the intertidal. Elevated temperature and gonad occupation were the strongest predictors for mortality. Selective plating for Vibrio spp. identified the pathogen V. aestuarianus from 24 of 180 bacterial isolates, based on sequence data for the RecA gene. This gram-negative pathogen has been described as a causative agent of summer mortality events in France. Future work will use next-generation sequencing to examine the changes in the microbiome throughout a juvenile mortality event, based on the V4 region of the 16S rRNA gene.
This project will provide the shellfish aquaculture industry with evidence of factors driving summer mortality in British Columbia, which will enable them to develop potential mitigation measures to lessen the impacts.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Mac’s Oysters Ltd.
Project Leader: Chris Pearce (DFO)
Project Team: Will Hintz, Paul de la Bastide, Terrie Finston (U Vic); Terri Sutherland (DFO)
Collaborators: Robert Marshall, Gordy McLellan (Mac’s Oysters Ltd.)
Contact: Chris.Pearce@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-P-05-eng.html

Oyster culture rafts at one of the study sties in Baynes Sound
Photo: Malcolm Cowan (DFO)
Assessment of factors leading to oyster mortality in Tracadie Bay (NB) and development of physical and biological tools for management
In August 2016, significant mortality of up to 75% of suspended oysters in some leases occurred in the northern branch of Tracadie Bay. Field observations were consistent with an anoxic event (i.e., water conditions characterized by an absence of dissolved oxygen and elevated hydrogen sulfide levels) caused by the eutrophication of the ecological system through an increase in nutrient concentration. As eutrophication is a major threat globally, it is important to increase knowledge and understanding of how productivity of cultured stocks might be impacted by the ecosystem in which they exist, and of how aquaculture operations impact the ecosystem.
The objectives of this project are to: 1) develop a hydrodynamic model capable of reproducing current flow patterns within Tracadie Bay; 2) determine areas within the bay that are at risk of hypoxia; and 3) determine thresholds of effect of anoxia, hydrogen sulfide and temperature on cultivated oysters using a combination of field and laboratory work. Together, field and laboratory work will provide powerful tools for managing Tracadie Bay and other shellfish aquaculture areas for the foreseeable future. The hydrodynamic model, in conjunction with the spatial assessment of organic content and benthic flux, will help identify areas at risk of nutrient-induced impacts.
While the capacity to predict areas at risk of impact is useful for management, it is also directly applicable for shellfish growers and their operations. Ultimately, identifying thresholds of oyster survivorship will provide baseline information for early warning signs and, potentially, indicators for growers to take action in the event of a future anoxic event. Findings from this work will also be of relevance for other fixed-gear aquaculture around the world, resulting in an increase in scientific capacity of the aquaculture industry.
Date: May 2017 – Dec. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: L’Étang Ruisseau Bar Ltd.
Project Leader: Michael Coffin (DFO)
Project Team: Luc Comeau, Thomas Guyondet, Michael Coffin (DFO); Rémy Haché, Sylvio Doiron (DAAF); Joannie Thériault (Commission of the Environment for the municipality of Tracadie); Jose Babarro (Instituto de Investigaciónes Mariñas CSIC); Ramon Filgueira (Dalhousie U); Michael van den Heuvel (UPEI)
Collaborators: André Mallet (L’Étang Ruisseau Bar Ltd.)
Contact: Michael.Coffin@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-G-02-eng.html

Randomly selected Eastern Oysters (Crassostrea virginica), separated by treatment, for a multi-factorial experiment on the effect of bacteria on median lethal time (LT50) under anoxic conditions.
Photo: M. Coffin (DFO)

A multi-factorial experiment on the effect of bacteria and anoxia on mortality of Eastern Oysters (Crassostrea virginica).
Photo: M. Coffin

Eastern Oysters (Crassostrea virginica) within anoxic and oxic chambers to assess median lethal time (LT50).
Photo: M. Coffin (DFO)

Eastern Oysters (Crassostrea virginica) within anoxic and oxic chambers to assess median lethal time (LT50).
Photo: M. Coffin (DFO)
Oyster aquaculture in an acidifying ocean: Effects of ocean acidification on Eastern Oysters (Crassostrea virginica) and the mitigation potential of seagrass (Zostera marina)
Ocean acidification (OA) – the decrease in oceanic pH and associated changes in the marine carbonate system resulting from increased oceanic uptake of atmospheric carbon dioxide (CO2) – poses a threat to marine life, as shifts in oceanic pH can have negative physiological and behavioural consequences for a wide array of marine biota.
This research will monitor the pH and carbonate chemistry in St-Simon Bay and Chaleur Bay, New Brunswick, and assess the biological effects of ocean acidification and ocean warming on hatchery-reared Eastern Oysters (Crassostrea virginica) at different stages of production. As well, it aims to determine the pH (and carbonate chemistry) buffering capacity of seagrass (Zostera marina) and explore seagrass as a potential mitigation strategy against OA.
This project will provide valuable information on OA in the coastal zone of Atlantic Canada, identifying how OA impacts oysters in their early life history stages, and developing a sustainable and environmentally-friendly mitigation strategy against OA. This project also implements research to support the use of emerging technologies to identify or alleviate associated environmental concerns, and to create more environmentally sustainable operations. Collectively, these research objectives will provide effective management strategies for oyster hatcheries in the advent of a detrimental global change stressor.
Date: Mar. 2017 – Mar. 2020
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: L’Étang Ruisseau Bar Ltd.
Project Leader: Luc Comeau (DFO)
Project Team: Claire Carver, Martin Mallet (L’Étang Ruisseau Bar Ltd.); Jeff C. Clements (Norwegian University of Science and Technology); Élise Mayrand, Sébastien Plante (Université de Moncton)
Collaborators: André Mallet (L’Étang Ruisseau Bar Ltd.)
Contact: Luc.Comeau@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-G-04-eng.html

Experimental setup at L'Étang Ruisseau Bar Ltd.
Photo: Martin Mallet (ERB)
Impacts of aquaculture operations on the genetic health of natural populations of the Eastern Oyster, Crassostrea virginica
By developing a new method to extract DNA from oysters and applying next-generation sequencing methods, this project demonstrated that New Brunswick oysters show between-population genetic differences. Hatchery experiments suggest that population differences could be commercially significant.
Wild oysters were collected from eleven sites in NB, as well as one site in PEI and one site in NS. A series of between- and within- population crosses were then performed. Although there was no evidence of local adaptation, and hybrids did not generally outperform their parents either, multiple differences in population traits were found.
Next generation sequencing methods were used to determine that at least four distinct oyster populations are present in New Brunswick. Briefly, oysters from northern NB form a single group, whereas oysters from Central NB appear to form two distinct genetic groups in the Miramichi and Richibucto areas, respectively. Finally, oysters from southern NB form a single group. All Gulf-region oysters appear more closely related to each other than to oysters from Cape Breton, NS. Levels of genetic diversity were similar across genetic groups.
The observed genetic diversity and differences in life-history traits open the possibility of improving oyster performance by selective breeding and can inform conservation practices.
Date: Apr. 2013 – Jun. 2017
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: L’Étang Ruisseau Bar Ltd.
Project Leader: Mark LaFlamme (DFO)
Project Team: Jean-René Arseneau, Royce Steeves (DFO); Martin Mallet (L’Étang Ruisseau Bar Ltd.)
Collaborators: André Mallet (L’Étang Ruisseau Bar Ltd.)
Contact: Mark.LaFlamme@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/G-13-01-001-eng.html

Oyster family being evaluated in St-Simon Bay.
Photo: Martin Mallet (L'Étang Ruisseau Bar Ltd.)

Broodstock oyster in conditioning tank.
Photo: Martin Mallet (L'Étang Ruisseau Bar Ltd.)
A tool for assessing the maximum sustainable production levels of Blue Mussels and Eastern Oysters in PEI
In Prince Edward Island (PEI), oyster aquaculture is gradually evolving from the traditional use of the benthic, on-bottom culture, to suspended, off-bottom culture. From a farming perspective, there are several advantages to suspending oyster stocks in the upper water column, such as protecting stocks from losses by benthic predators. From an ecological point of view, the removal of phytoplankton by farmed oysters must not surpass the capacity of the ecosystem to replenish the supply of phytoplankton, since this could result in adverse conditions for other grazers in the system, such as zooplankton and wild shellfish.
This project will investigate aspects of carrying capacity parameterization that are poorly understood. In particular, the project will focus on the functioning of aggregate oysters in the floating oyster bag (or cage) and determine the rates of primary production in key bays where oyster farming development may occur. Using this new information, the capacity of future farm bivalves to pump water (Clearance Time, CT) will be compared against the capacity of the systems to replenish the phytoplankton (Primary Production Time, PT). Results will provide insight into sustainable bivalve production levels for aquaculture managers in PEI.
Date: Apr. 2018 – Mar. 2020
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Luc Comeau (DFO)
Project Team: Thomas Guyondet, Rémi Sonier, André Nadeau (DFO)
Collaborators: Ramon Filgueira (Dalhousie U); Réjean Tremblay (UQAR); Jeff Davidson, Jonathan Hill (AVC)
Contact: Luc.Comeau@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2018-g-02-eng.html

Collecting water samples for the measurement of primary production.
Photo: Jonathan Hill (AVC)

Measurement of primary production by injecting a stable isotope tracer.
Photo: Jonathan Hill (AVC)
The impacts of pre-winter conditioning on the survival, growth, and reproductive yield of the Eastern Oyster (Crassostrea virginica), cultivated in suspension
The Eastern Oyster (Crassostrea virginica) is subjected to pronounced seasonality at its northernmost distribution limit in Atlantic Canada. After spawning in July, oysters feed intensively until November in order to prepare for reproduction and the build-up of energy reserves. This process is called pre-winter conditioning. Oysters resume feeding in the spring and the bulk of their energy intake is seemingly allocated towards shell formation.
This study provides the first quantitative assessment of oyster death resulting from inadequate pre-winter conditioning. Poorly conditioned oysters died nearly three times more frequently than control oysters, and those that survived winter remained in poor condition during the ensuing spring and summer. Despite their vulnerable state, poorly conditioned oysters invested as much energy in reproduction as the healthier control oysters. Oysters exhibited very little growth over the duration of the study and therefore shell height was similar for poorly conditioned and control oysters.
The aquaculture industry has control over husbandry practices that are known to impact pre-winter conditioning. It is estimated that poor-husbandry practices would result in the death of 24% of oysters, versus 9% under ideal conditions. Improved practices that will increase oyster pre-winter conditioning could therefore greatly increase the yield of the aquaculture industry.
Date: Apr. 2015 – Mar. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: L2 - Recherche et Production Aquacole Inc.
Project Leader: Luc Comeau (DFO)
Project Team: Denise Méthé, Michelle Maillet (DFO)
Collaborators: Léon Lanteigne (L2 - Recherche et Production Aquacole Inc.)
Contact: Luc.Comeau@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-G-01-eng.html

Columns of trays holding cultivated oysters, Crassostrea virginica.
Photo: Léon Lanteigne (L2 - Recherche et Production Aquacole Inc.)
Interactions between oyster farming and the productivity of wild oyster beds
In Prince Edward Island, Eastern Oyster (Crassostrea virginica) farming relies almost entirely on natural spat produced by wild oyster beds. In the oyster life cycle, free-floating larvae settle on and attach to a hard surface, a stage known as spat, before developing into juvenile oysters. Presently, there are 226 spat collection (fishing) licenses and the demand for additional licenses is progressively growing with the development and expansion of the oyster aquaculture industry. The demand for oyster spat may be impacting natural settlement and recruitment processes on wild oyster beds.
This project will investigate the preferences of oyster larvae to man-made and natural seabed substrates for settlement. The experiment will be conducted with the Lennox Island First Nation, which operates an oyster hatchery, and researchers at the University of Prince Edward Island (Atlantic Veterinary College) and the Université de Moncton. Oyster larvae from both wild and cultured oysters will be exposed to three man-made and three natural substrates, with and without substrate choices, to determine the oyster recruitment to different substrates. Results will provide a preliminary understanding of the dynamics between wild and farmed oysters and will inform management decisions regarding the use of artificial spat collectors.
Date: Mar. 2017 – Apr. 2018
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Luc Comeau (DFO)
Project Team: Anne-Margaret MacKinnon, Luke Poirier, John D.P. Davidson (DFO); Randy Angus, Steve Palmer (Bideford Shellfish Hatchery); Jeff Clements (NTNU); Gilles Miron (U Moncton); Jonathan Hill, Jeff Davidson (AVC)
Contact: Luc.Comeau@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-g-10-eng.html

Project workshop at the Atlantic Veterinary College in Charlottetown, PEI brought scientists from around the world together with various stakeholders from the Gulf Region. From left: Chris Mills, Jarrod Gunn McQuillan, Pedro Quijon, Thomas Landry, Gilles Miron, Randy Angus, Franck Lagarde, Kim Gill, Thomas Guyondet, Aaron Ramsay, Luke Poirier, Marie-Josée Maillet, Luc Comeau, Thitiwan Patanasatienkul, Jeffery Clements, and John Davidson (host Jeffrey Davidson was absent during photo).
Photo: Rémi Sonier (DFO)
Production of oyster seed adapted for sea ranching
In Canada, shellfish culture techniques currently used can be divided into two categories: off-bottom and bottom culture. Off-bottom techniques are used in most commercial oyster production in New Brunswick and in the neighboring province of Prince Edward Island. However, most of the leases granted are bottom culture leases, which are noticeably underutilized as the seed available do not meet the specific requirements of this environment given current culture techniques. Predation is the main underlying reason for this underutilization.
This research aims to develop an adapted seed production approach to produce seed especially well-suited to sea ranching, and to establish a tailor-made seeding procedure to minimize the risk of predation. The project also seeks to validate the targeted characteristics of the seed for seeding purposes, and the procedures for optimizing post-seeding survival rates. It also seeks to produce oysters with thicker shells to mitigate predation impacts such as the stress related to the aggressiveness of the crab, Cancer irroratus. The project will provide the first quantitative information on whether the risk of oyster predation can be reduced by increasing seed oyster shell length and thickness.
Consequently, understanding shell properties, in particular the foliated layer, can help us understand the defenses oysters use to survive predation. The mechanical defense provided by shells allows oysters to perform essential ecosystem services such as habitat formation, water filtration and benthic-pelagic coupling in marine and estuarine systems. In addition, the project will help develop approaches for managing issues relating to the health of aquaculture species.
Date: Apr. 2017 – Mar. 2020
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: L2 - Recherche et Production Aquacole Inc.
Project Leader: Rémi Sonier (DFO)
Project Team: Luc Comeau, Luke Poirier, André Nadeau (DFO)
Collaborators: Léon Lanteigne (L2 - Recherche et Production Aquacole Inc.)
Contact: Remi.Sonier@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-G-05-eng.html

Crab predation on oyster.
Photo: Luke Poirier (DFO)
Investigating Polydora outbreak in New Brunswick off-bottom cultured oysters
Known simply as a mudworm or blisterworm, Polydora websteri can bore into the shells of live and dead shellfish. Its presence among New Brunswick oyster populations has typically been minor and usually of low intensity. However, there have been sporadic increases of infestation rates observed in off-bottom oyster growing sites in New Brunswick, which could ultimately lead to serious impacts on oyster populations and result in economic losses for the aquaculture industry.
This study provides an assessment of parasitic mudworm infestations in Eastern Oysters cultured in Northern New Brunswick near the entrance of the Richibucto Estuary. According to a newly-developed visual rating guide, oysters cultured at sites closer to the entrance of the Richibucto Estuary showed more severe effects (i.e., more worm tunnels and mudblisters) than at sites further away from the estuary. While dead and live oysters both contained similar numbers of P. websteri, dead oysters displayed more severe effects of the parasite than live oysters. A strong negative relationship existed between the severity of P. websteri infestation and oyster condition index. Experiments also revealed that increased silt accumulation on live oysters can accentuate the number of P. websteri that recruit on a given oyster, and that exposing empty oyster shells to low pH conditions for three to five weeks can reduce the number of P. websteri recruiting to a given shell.
Collectively, the results of this study show that P. websteri reduces oyster condition index in off-bottom cultured oysters and increases susceptibility to mortality. Keeping oysters clean of silt and sediment, or exposing oysters to low pH may reduce the severity of the infection. The results could also inform the geographic placement of oyster farms in the future.
Date: Apr. 2014 – Mar. 2017
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Huîtres Aquador Oysters Inc.
Project Leader: Daniel Bourque, Mary Stephenson (DFO)
Project Team: Janelle McLaughlin, Jeff Clements (DFO)
Collaborators: Serge Gaudet (Huîtres Aquador Oysters Inc.)
Contact: Daniel.Bourque@dfo-mpo.gc.ca, Mary.Stephenson@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/G-14-01-002-eng.html
Improving physiological health of oysters by selecting seed for stress resilience
Access to a consistent supply of high quality and resilient seed stocks has been identified as a key constraint for continued viability and expansion of the Eastern Oyster industry in Atlantic Canada.This project was focused on generating efficient and resilient oysters to ensure that, if faced with a pathogen or environmental stressor, they will have an increased capacity to launch an immune response, while increasing growth and feed efficiency performance.
Absorption efficiency and metabolic rate (aerobic capacity) were measured, from two sources of oysters from Bouctouche, NB estuary (wild and aquaculture). In total, seventeen genetic markers that correlated with absorption efficiency and ten with metabolic rate in both optimal and stressful conditions were identified.
Four spawning groups were then developed from broodstock with genetic markers that were identified as being linked to high and low growth rate and feed efficiency. Each group was then spawned and the new generation was grown in hatchery conditions before being transferred to the grow-out site in Bouctouche Bay, NB, and sampled at approximately one year of age. Overall, no significant differences were found between spawning groups in terms of growth. However, growth was highly variable among individuals, indicating a large selection potential. The objective is that the genetic markers identified in this project will ultimately allow for the selection of desirable phenotypes that will improve stock performance.
Novel genetic markers correlated to absorption efficiency and metabolic rate were discovered in this study. This discovery allows for further study to be done, to enhance our knowledge of physiological aspects of resiliency, growth and feed efficiency. Additionally, it allows opportunity for continued improvement of oyster stock in the future, and increase viability of the industry.
Date: Jan. 2015 – Jun. 2017
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: L’Écloserie Acadienne Ltd.
Project Leader: Denise Méthé (DFO)
Project Team: Sarah Stewart-Clark, Stephanie Hall (Dalhousie U); Gillian Tobin-Huxley (DFO); Fraser Clark (Mount Allison U)
Collaborators: Maurice Daigle (L’Écloserie Acadienne Ltd.)
Contact: Denise.Methe@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/G-14-03-001-eng.html

Gillian Huxley-Tobin (DFO) sieving oyster spat produced from selected broodstock.
Photo: Denise Méthé (DFO)
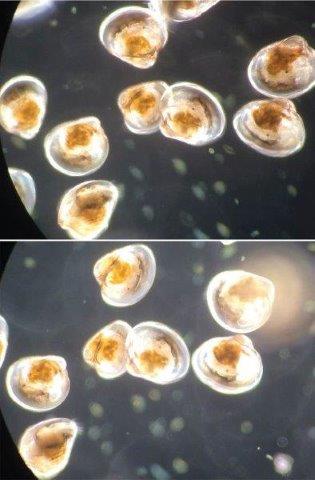
Eastern Oyster larvae with eye spot.
Photo: Stephanie Hall (Dalhousie U)
Shellfish: Other
Understanding the distribution of a nemertean predator, Cerebratulus lacteus, in clam flats: Implications for control measures
Clams could be an important species for the future development of aquaculture in Atlantic Canada. One of the major obstacles in the development of clam culture has been controlling predators on culture sites. In recent years, commercial size clam densities have been reportedly lower. While the cause for these declines has not yet been documented, harvesters have noted the presence of predatory worms at clam harvesting sites. The milky ribbon worm (Cerebratulus lacteus) is an important predator of many bivalve species living within the sediment on the seafloor, and its presence has been related to high field mortality in Soft-shell Clams. Very little, however, was known about the factors regulating the patchy distribution of this predator.
The results of this project ultimately provided evidence for milky ribbon worm predation on Soft-shell Clams and Razor Clams in Atlantic Canada. The experiment suggests that the milky ribbon worm likely forages by selecting prey species that provide the most energy per unit of foraging time. This approach is evidenced by selective predation on the more profitable Razor Clams when provided a choice of Razor Clams and Soft-shell Clams. While predation rates are relatively low, the milky ribbon worm is gregarious and may exert population level impacts when occurring at high densities.
By describing the predation of C. lacteus on infaunal clams in Atlantic Canada, the results can aid in site selection for commercial clam operations. It also provides a baseline estimate for predation rates of C. lacteus on bivalve prey.
Date: Apr. 2014 – Jun. 2017
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Innovative Fisheries Products Inc.; Mills Seafood Ltd.
Project Leader: Luc Comeau (DFO)
Project Team: Daniel Bourque, Angeline LeBlanc, Jeffery Clements (DFO)
Collaborators: Doug Bertram (Innovative Fisheries Products Inc.); Tim Williston (Mills Seafood Ltd.)
Contact: Luc.Comeau@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/G-14-01-001-eng.html

Nemertean Cerebratulus lacteus
Photo: Daniel Bourque (DFO)

Juvenile oyster seed in culture trays in Baynes Sound, British Columbia. Photo: Terri Sutherland (DFO)
Scaling hatchery methods for the commercial production of land based sea urchin farming in New Brunswick
Quoddy Savour Seafood Ltd. is a New Brunswick supplier of premium grade sea urchin roe with a desire to be self-reliant from collection of wild-caught sea urchins. Much is already known related to closing the sea urchin lifecycle given its prominence in education and toxicology settings. However, scaling these principles in a commercial operation has not been accomplished in Canada to date. This project endeavors to commercialize sea urchin closed cycle hatchery production to meet project specific objectives associated with target numbers of post-settled sea urchins produced. Specific activities to date include:
- Collecting candidate sea urchin broodstock from the local fishery, then returning these individuals to the Huntsman wet laboratory facilities;
- Enhancing, and then long-term holding, adult sea urchins to provide high quality male and female gametes in culture conditions year round;
- Inducing release of male and female gametes and fertilizing eggs for the next generation of thousands of sea urchins for grow-out;
- Incubating thousands of fertilized eggs prior to transfer of hatched individuals;
- Producing multiple species of microalgae in sufficient quantity to offer a balanced diet to planktonic larvae;
- Conditioning settlement substrate and exploring options to collect competent sea urchins following settlement; and
- Growing juvenile sea urchins through the post-settlement stage until individuals are of a sufficient size deemed appropriate for transfer to the commercial facility.
Delivery of the initial thousands of postsettled juvenile sea urchins is targeted for the end of 2018 for further growth at the Quoddy facility in Pennfield, NB, until eventual commercial sales at the appropriate size.
Success from this project will decouple the Quoddy operations from wild captured sea urchins and therefore allow sale of premium grade product year round. Hatchery production will also present opportunities to eventually capitalize on quantitative genetic selection and, therefore, production of consistent grade product for sales to the market in less time. Finally, closed hatchery production, and possibly establishing a future genetic pedigree, will provide product traceability for global sales, thereby eliminating concerns related to sale of product during closed wild fishery seasons.
Date: Oct. 2017 – Jun. 2019
Funded by: Quoddy Savour Seafood Ltd.
Co-Funded by: NBIF
Project Leader: Anne McCarthy, Amber Garber (HMSC)
Project Team: Cailey Dow, Rebecca Eldridge, Elizabeth Dowling, Sarah Rogers, Sarah Gravel, Esther Keddie, Chris Bridger (HMSC)
Collaborators: Roger Waycott, Chad Brown, Rob Bosien (Quoddy Savour Seafood Ltd.); WD Robertson (ReThink Inc.)
Contact: amber.garber@huntsmanmarine.ca
Website: Huntsmanmarine.ca
The effects of a hydraulic dredge and adding shells on the environment and Soft-shell Clam population dynamics
To improve the profitability of Soft-shell Clam farms in Atlantic Canada, growers want to use hydraulic dredging as a means of harvesting clams. However, concerns regarding mechanical clam harvesting include shell damage, impacts on recruitment and survival of juveniles, impacts on biodiversity, and the release of buried organic matter and reduced metabolites. Without an understanding of these issues, it is not clear whether hydraulic dredging is a sustainable harvesting method.
Given that both sediment geochemistry and clam harvesting can potentially affect clam populations, we conducted two separate studies to determine: 1) the effect of clam dredging on sediment pH and the abundances of two bivalve species (juvenile Mya arenaria and adult Limecola balthica) within and between locations, and 2) the effect of sediment geochemistry on the abundance of the same bivalves within a small spatial area.
While differences between locations were evident in this study, clam abundances did not appear to be impacted by dredging at most locations, with the exception of one site which had higher clam abundances in dredged plots. Clam abundances did not appear to correlate with sediment geochemistry. This suggests that, where harvesting might affect clam abundances, the physical process of dredging rather than the indirect effects on sediment geochemistry likely drive clam abundance patterns.
Date: Oct. 2015 – Jun. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Mills Seafood Ltd.
Project Leader: Angeline LeBlanc (DFO)
Project Team: Jeffery Clements, Luc Comeau (DFO)
Collaborators: Marilyn Clark (Mills Seafood Ltd.)
Contact: Luc.Comeau@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-2-G-02-eng.html
Health status update of Mercenaria mercenaria in St. Mary's Bay, Nova Scotia
With the rise of climate change, many pathogens have changed latitudinal ranges and have become invaders of new ecosystems where they have the potential to cause high mortality rates. Fundamentally, understanding shellfish habitat and how shellfish are vulnerable to change is a factor that needs close attention to allow the development of a better understanding of the effects of climate change and the implications for shellfish aquaculture.
This project will revisit the health status of the Quahog (M. mercenaria) and environmental conditions in St. Mary’s Bay, and will add measures pertaining to shell condition. It will also measure calcium carbonate (aragonite) availability, upon which the Quahog is dependent, and it will aim to compare the health and shell conditions between sites within the sampling area.
Improving the knowledge base about diseases and other climate change stressors will be important for future monitoring of this ecologically and economically important area, and for mitigation purposes.
Date: May 2017 – Apr. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Innovative Fisheries Products Inc.
Project Leader: Michelle Maillet (DFO)
Collaborators: Doug Bertram (Innovative Fisheries Products Inc.)
Contact: Michelle.Maillet@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-G-01-eng.html

Intertidal flats of St. Mary’s Bay, Nova Scotia.
Photo: Michelle Maillet (DFO)

Shell of Mercenaria mercenaria.
Photo: Michelle Maillet (DFO)
Microplastics and shellfish aquaculture: Presence, extent, potential impacts, and mitigation measures
Microplastic particles (MPs) are plastic particles <5 mm in their longest dimension, and they contaminate habitats worldwide. This project assessed the extent, impact, and potential mitigation measures related to MP contamination of BC shellfish aquaculture species and their environment.
We assessed the MP concentrations in Pacific Oysters and Manila Clams outplanted for two to three months at eleven intertidal shellfish farms and ten non-aquaculture sites in southern BC. We found that average contamination of shellfish was less than one MP/individual and no significant difference existed between shellfish grown on or off shellfish farms. Spectroscopic analysis of some of the MPs found in the shellfish and in the water-column suggested they likely came from textiles such as nylon and polyester. We also compared intertidally farmed and raft-grown oysters in three areas and found no significant difference in MPs between the farming methods. In the laboratory, we exposed Pacific Oysters to environmentally relevant concentrations (5 fibres/L) and types (acrylic, nylon, polyester, and polypropylene/polyethylene) of MPs over a 30-day period. No significant differences were observed in condition index or lysosomal membrane stability compared with controls not fed MPs. However, MP ingestion led to an upregulation in physiological pathways associated with immunity and stress and a downregulation in pathways associated with reproduction, suggesting potential long-term negative consequences of MP ingestion. We further conducted a depuration experiment and determined that MP load could be reduced after five days in a clean environment, but suggest that the procedures required to create an MP-free environment may not be feasible for adoption by bivalve depuration facilities.
The results of this study suggest that the plastic equipment used in shellfish aquaculture does not appear to increase the risk of cultured shellfish ingesting MPs relative to wild counterparts. Nevertheless, efforts on all fronts should be made to reduce the contamination of the environment with microplastics, especially from textiles, which are commonly released via sewage effluent, aerial dispersal, and from in situ degradation of synthetic ropes.
Date: Sep. 2015 – Mar. 2018
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: British Columbia Shellfish Growers Association (BCSGA)
Project Leader: Sarah Dudas, Helen Gurney-Smith (VIU/DFO); Chris Pearce (DFO)
Project Team: John Dower, Garth Covernton, Monique Raap (U Vic); Peter Ross (Ocean Wise); Matt Miller, Maggie Dietterle (VIU)
Collaborators: Darlene Winterburn (BCSGA)
Contact: Sarah.Dudas@dfo-mpo.gc.ca; Helen.Gurney-Smith@dfo-mpo.gc.ca; Chris.Pearce@dfo-mpo.gc.ca
Website: www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-P-01-eng.html

Oyster up close.
Photo: Kayla Mohns (DFO)

Netting.
Photo: Kayla Mohns (DFO)

Various microplastic fibres under microscopy.
Photo: Garth Covernton, Monique Raap (U Vic)
Effects of a prepared diet and kelp on gonad enhancement in the green (Strongylocentrotus droebachiensis) and red (S. franciscanus) sea urchins
Sea urchins are ecologically-important keystone predators that can drastically alter marine communities due to their consumption of macroalgal beds, creating areas termed “urchin barrens”. Sea urchins in these barrens generally have low, nonmarketable gonad (roe) yields. Sea urchin gonad enhancement entails removing low-gonad-yield individuals from these urchin barrens, which would otherwise not be removed by the fishery, and feeding them prepared/ natural diets that can bulk up their gonads to market size in eight to twelve weeks. Previous studies have achieved large gonad yields using various prepared diets, but none have consistently obtained market quality taste and colour (bright orange/yellow). This study aimed to improve gonad yield and quality in green (S. droebachiensis) and red (S. franciscanus) sea urchins using a newly developed sea urchin pellet feed. The sea urchins were held in specifically designed sea urchin trays at a sea-based farm site in Departure Bay, Nanaimo, British Columbia and fed either the prepared diet or bull kelp (Nereocystisluetkeana) for twelve weeks. While the prepared diet did significantly increase gonad yield in S. droebachiensis, the kelp diet produced better gonad quality, as has been seen in previous studies.
Further research will examine the impact of three temperatures (8, 12, and 16ºC), two new prepared diets, and kelp on gonad enhancement in S. droebachiensis and S. franciscanus in a land-based system. Analysis of feed and faecal composition will also be conducted and used along with oceanographic data to examine the potential environmental impact a commercial sea-based farm would have using the waste deposition model DEPOMOD.
As the aquaculture industry continues to rapidly grow worldwide, it is critical to understand the impact the farmed species and diets could have on the surrounding environment. The results of these studies will be used to ensure that sea urchin aquaculture can become both an environmentally and economically sustainable new industry in British Columbia.
Date: Jan. 2017 – Apr. 2020
Funded by: Fisheries and Oceans Canada (DFO)
Co-Funded by: Urchinomics; Nova Harvest Ltd.; U Vic
Project Leader: Chris Pearce (DFO)
Project Team: Steve Cross (U Vic); Stefanie Duff, Emily Warren, Julia Savery (VIU); Lyanne Curtis (DFO)
Collaborators: Brian Takeda, Harm Kampen (Urchinomics); J.P. Hastey (Nova Harvest Ltd.)
Contact: Chris.Pearce@dfo-mpo.gc.ca

Red sea urchins in a tray.
Photo: Emily Warren (VIU)
Developing a carrying capacity framework for Baynes Sound, B.C. – Recent findings regarding the hydrodynamic influence on seabed characteristics
A framework for a shellfish carrying capacity assessment includes the development of a high-resolution, spatially-explicit hydrodynamic model (e.g., Finite Volume Community Ocean Model (FVCOM)). This model supports baywide assessments of pelagic and seabed characteristics to support management decision-making with respect to shellfish aquaculture facilities.
In terms of seabed characterization, sediment texture was related to modelled maximum velocity values within 5 m of the seabed (Umax,5) where the highest values (restricted southern entrance) and lowest values (northern deep basin) represented sand-depositional and muddepositional facies. The strong hydrodynamic gradient influenced the geotechnical, biochemical and meiofauna attributes located along the central axis of Baynes Sound (2009–2014).
In general, sediment porosity, organic carbon and nitrogen content, and trace-element concentrations (e.g., zinc, copper) increased with increasing sediment fines towards the head of the Sound. In contrast, sediment pore-water sulfide maintained an oxic category (0-700 μM) for the most part and exhibited a lack of variation within the Sound. Finally, PCA results revealed that meiofauna were associated with a medium sand fraction (0.25 mm) characterized by lowporosity and organic sand-loam textures.
In terms of characterizing the pelagic environment, data are currently being analyzed to examine spatial and temporal fluctuations on a bay-wide scale.
Date: Sep. 2011 – Mar. 2017
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO–PARR)
Co-Funded by: Ecosystem Research Initiative (2009–2010)
Project Leader: Terri Sutherland (DFO)
Project Team: Lorena Garcia-Hoyos, Maxim Krassovski, Michael Foreman, Perry Poon (DFO); Alan Martin (Lorax Environmental Services); Carl Amos (Southampton University)
Contact: Terri.Sutherland@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2011-P-21-eng.html
Miscellaneous
Study of the spread of cultivated kelp around marine farms
In Québec, kelp cultivation is a young industry. Currently, all of Québec’s seaweed culture companies use sugar kelp (Saccharina latissima) seedlings from Bonaventure’s broodstock on marine farms of the Gaspé Peninsula and the North Shore. During the cultivation on farms, self-thinning occurs on culture lines. It is likely that loosely attached seedlings fall off the line because of competition, currents or during bad weather, and the outcome of these seedlings is not documented. Presently, there is no information on the genetic structure of the kelp populations in the Gulf of St. Lawrence and it is not known if wild beds from different regions are genetically distinct. Yet, seaweed farmers need to identify and select the strains with the best characteristics. It is therefore important to address these questions to help them make evidence-based decisions and to support the sustainable growth of their companies.
This project aims to investigate the possible introduction of Bonaventure strain on various sites in Québec, such as the Sept-Îles and Paspébiac sites, and to study the possible dispersal of the cultivated strain around the cultivation site. In addition, a project funded by the FAP UQAR-Merinov and the Optimal program (NSERC) will establish the genetic structure of sugar kelp populations in Quebec, in order to determine the genetic variation between the local and Bonaventure strains.
It is intended that the results of this project will be able to inform decision makers on the possible dispersal of cultivated sugar kelp (Bonaventure strain) around various algoculture sites in Québec.
Date: Jan. 2018 – Mar. 2020
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-funded by: Ferme Maricole Purmer; The Mi’gmaq Maliseet Aboriginal Fisheries Management Association (MMAFMA)
Project Leader: Rénald Belley (DFO)
Project Team: Mathilde Lemoine, Isabelle Gendron-Lemieux, Marie-Pierre Turcotte (Merinov); France Dufresne, Frédérique Paquin (UQAR)
Collaborators: Marie-Hélène Rondeau (MMAFMA); Sandra Blais (Ferme Maricole Purmer)
Contact: Renald.Belley@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm

Cultivated sugar kelp (Saccharina latissima) on a culture line at an aquaculture site.
Photo: Isabelle Gendron-Lemieux (Merinov)
Reproduction and cultivation of the red algae Palmaria palmata (palmariales, rhodophyta) in the Gulf of St Lawrence, Canada
Palmaria palmata is an edible red seaweed of the North Atlantic. In Eastern Canada, it is mainly harvested around Grand Manan Island and cultivation can be seen as a desirable alternative to relieve the pressure on wild stocks. Its commercial cultivation, however, is neither mastered nor practiced in Canada. The objectives of this project were to: a) test a method to artificially induce sporogenesis on fronds; b) compare different culture parameters and substrates seeded with tetraspores; and c) optimize the growth of fronds cultivated in tanks.
Wild fronds were harvested locally and non-fertile fronds were cultivated at 5 and 10°C under either a constant long or short photoperiod. Braided nylon ropes, stranded kuralon rope, plastic nets and non-woven artificial textiles were seeded with spores, cultivated in tanks and the distribution and number of thalus were measured. Young fronds were cultivated in tanks with various combinations of stocking density, temperature, light intensity and nitrogen concentrations. Fresh biomass was measured weekly and the productivity per unit surface of tank was calculated.
The preliminary results showed that, when non-fertile fronds are artificially conditioned, mature spores are only produced at 5°C under a constant short photoperiod after two months. There was a higher density (1.2 ± 0.5 thallus per cm) of plants on kuralon ropes and on the At-Sea textile (2.9 ± 1.7 thallus per cm2), but some individuals were loosely attached on these substrates. In the culture tanks, the highest productivity was obtained with young fronds, grown at 2.5 kg m-2 with nutrient enrichment.
The next steps will be to transfer the seeded substrates to a marine farm, to perform organoleptic tests on the cultivated fronds and to scale up the culture technology in a private marine hatchery. The project is designed to ultimately support the diversification and increase the profitability of the seaweed aquaculture industry.
Date: Apr. 2017 – Apr. 2020
Funded by: Fonds de Québécois de Recherche Nature et Technologies (FRQNT)
Project Leader: Éric Tamigneaux (Merinov)
Project Team: Mathilde Lemoine, Jean-Claude Blais, Grégoire Cholat-Namy (Merinov); Arthur Kaufling, Tristan Reesor (Cégep de la Gaspésie et des Îles)
Contact: Etamigneaux@merinov.ca
Website: http://www.merinov.ca

Mathilde Lemoine harvesting dulse (Palmaria palmata) in Grande-Rivière, Québec.
Photo: Merinov, 2018.
Development of a red algae (Porphyra sp.) biological filter to reduce nitrogen in American Lobster tank effluent
In processing plants, live lobsters are kept in summer in fish tanks supplied with cold seawater. To reduce cooling costs, some of the water is reused so that the system is in partial recirculation. The ammonia that American Lobsters (Homarus americanus) secrete through their gills therefore accumulates in the water and can increase the mortality rate of animals. Some red algae of the family Bangiaceae (Porphyra sp., Pyropia sp.), also called nori, have high growth rates in cold water and a high capacity for nitrogen absorption and sequestration. In addition, nori is grown on a large scale in Asia and this seaweed has a high value in the food market. The algae culture and processing companies are interested in marketing native nori species and need access to a stable and sustainable supply source.
The present project aims to develop a biological filter based on native nori species to reduce the total nitrogen concentration in the effluents of the processing plants that hold live lobsters in tanks. The secondary objectives are: (1) to determine which of the local species of nori has the best potential for the uptake and sequestration of dissolved ammonia; (2) ensure the availability of good quality young fronds to seed the biofilter; (3) develop and test two biofilter prototypes with fixed or free fronds; (4) put the best-performing biofilter prototype on a pilot scale; (5) establish the nutritional and organoleptic value of nori fronds produced in the biofilter; and (6) establish operating costs and profitability of the biofilter.
The development of a biofilter to treat effluent would allow seafood plants to increase the volume of recirculated effluent, reduce cooling costs, and reduce mortality rates in fish tanks. For companies already practicing seaweed farming and brown algae processing, the project represents an opportunity to obtain important information on the life cycle, culture and nutritional value of Porphyra sp. This supports their efforts to diversify the supply of algae-based food products.
Date: Jun. 2019 – Jun. 2020
Funded by: Québec Ministry of Higher Education and Training — Research and Transfer Assistance Program (MÉES – PART)
Project Leader: Lisandre Solomon (Merinov)
Project Team: Isabelle Gendron-Lemieux, Éric Tamigneaux (Merinov)
Contact: Lisandre.Solomon@merinov.ca
Website: http://www.merinov.ca

American Lobsters (Homarus americanus) in a holding tank.
Photo: Jean-François Laplante (Merinov)
Completion of cross-cutting aquatic research to support aquatic broodstock, animal health and toxicology studies
Huntsman has completed several proprietary studies within upgraded facilities related to broodstock development, aquatic animal health and aquatic toxicology. Specific examples include:
- Atlantic Salmon broodstock-related sea lice infection challenges to measure degrees of family resistance;
- Acceptance of new feed and pharmaceutical ingredient formulations within study populations;
- Residue depletion studies involving new candidate drugs and various tissue analysis following numerous days post-dosing;
- Simulated commercial hatchery comparison studies testing various environmental and production parameters; and
- Atlantic Salmon choice of dissolved oxygen conditions.
These aquatic studies require controlled environments within multiple treatments and replicate tanks with volume to house numerous appropriately sized individuals. The dedicated Huntsman facility provides controlled access and includes the following capacity to ensure project-specific objectives are met:
- Eighteen independent tanks that house up to post-smolt sized (~400 g) Atlantic Salmon;
- Water temperature control (±1ºC) from 2-14°C at any time of the year;
- Tank-specific swirl separators to ensure fecal castings are collected for inspection and every uneaten feed pellet is captured to be noted within the study dataset;
- Ability to separate between different studies (i.e., different rooms, curtains or and/or practices); and
- Tank system alarms to provide study security.
Adding facilities that allow year-round temperature control within large-scale tank systems to the overall research capacity of the Huntsman is a game changer. Now commercial, government and academic studies, such as those listed, are completed as business requirements demand rather than seasonally when ambient conditions match study parameters. Added features of the systems, such as tank-specific swirl separators, ensure study fish are indeed acclimated by returning to feed post-transfer and allow monitoring of fish population feed consumption, which is critical when conducting fish health and treatment studies for regulatory purposes.
Date: Jan. 2017 – Dec. 2018
Funded by: Atlantic Canada Opportunities Agency–Atlantic Innovation Fund (ACOA–AIF)
Co-Funded by: New Brunswick Innovation Foundation
Project Leader: Chris Bridger (HMSC)
Project Team: Anne McCarthy, Phil Wiper, Amber Garber, Ehab Misk, Ben de Jourdan (HMSC)
Contact: chris.bridger@huntsmanmarine.ca
Website: Huntsmanmarine.ca

Completion of a study to determine residue depletion differences between post-smolt and adult Atlantic Salmon post-dosing.
Photo: Chris Bridger (HMSC)
Reducing the problem of early sexual maturation in Arctic Charr (Fraser River stock)
Early sexual maturation among diploid Arctic Charr and other farmed salmonids remains a costly problem, reducing meat quality and revenue. Photoperiod and food availability exert a strong influence on somatic growth and the decision to commence sexual maturation, but how they interact is unclear. Fraser River Arctic Charr is a good ’model salmonid’ for study as both sexes suffer a high rate (>70%) of early sexual maturation age 2+ in culture in 10°C groundwater, a trait that has limited its commercialization in Canada.
Reducing the incidence of maturity to <20% has been consistently achieved in four year classes by rearing fish under 24-h light (LL) from October to February. The timing of both the start- and end-date of LL has proved critically important. Further reductions in the maturity rate, to 0% in some cases, was achieved by combining LL with reducing somatic growth in winter by food deprivation. Food control alone, however, was less effective and reduced maturation among females only. Nonetheless, the mechanism by which the ’physiological decision’ to mature is switched off is influenced by both growth-independent and growth-dependent factors, for which we proposed a two-step gating mechanism.
In sum, this study found that Arctic Charr farms operating on flow-through well water at 9–10°C can reduce the incidence of early maturation in diploid Fraser River strain to <10%.
Date: Sep. 2013 – Dec. 2016
Funded by: Coastal Zones Research Institute, Shippagan (now Valorēs)
Co-funded by: ACOA Atlantic Innovation Fund
Project leader: Jim Duston (Dalhousie U)
Project team: Qi Liu (Dalhousie U)
Collaborators: Tony Manning (RPC)
Contact: jduston@dal.ca

Marketable silvery immature Arctic Charr (top) and unmarketable highly coloured mature fish (bottom).
Photo: Paul MacIsaac (Dalhousie U)
Biomarker platform for commercial aquaculture feed development
In this project, the partners have jointly developed multi-gene expression tools (i.e., multiplex qPCR) for assessing the health of Atlantic Salmon fed newly designed grower and clinical commercial feeds. Historically, the measurement of the performance of salmon feed in commercial fish trials was limited to growth rate metrics, but the development of tools for health monitoring in addition to performance is the next step for the production of novel, quality feeds. The objective was to develop and validate the use of this health and performance diagnostic platform in commercial feed development. Research outputs include:
- The development of over 350 unique assays to quantify the expression of functionally annotated biomarker genes.
- Multiplex panels were developed based on results obtained from extensive gene expression profiling studies (using microarrays, also called “gene chips”) conducted on various tissues collected in feeding trials. To select the biomarker genes for inclusion in the multiplex panels, single-gene qPCR and tissue lipid composition data, as well as growth performance data, were analyzed using several multivariate statistical methods.
- Multiplex qPCR panels developed in this project allow the simultaneous analysis of multiple biomarker genes capable of predicting feed performance in a quick and cost-effective manner. We have used these multiplex panels and complementary data (e.g., lipid/fatty acid analyses) to test salmon feed raw materials for impact on multiple physiological parameters such as growth, lipid metabolism, immune response, oxidative stress, and inflammation. These techniques can be scaled up as a routine practice to help Cargill Animal Nutrition in the design of novel growth-promoting and immune-boosting feeds.
Date: Oct. 2014 – Mar. 2018
Funded by: Genome Canada — Genome Applications Partnership Program
Co-Funded by: Cargill Animal Nutrition; InnovateNL; Atlantic Canada Opportunities Agency; Genome Atlantic
Project Leader: Matthew Rise (MUN); Richard Taylor (Cargill Animal Nutrition)
Project Team: Albert Caballero-Solares, Maryam Beheshti Foroutani, Xi Xue, Khalil Eslamloo, Tomer Katan, Nicole Smith, Sabrina Inkpen, Jeanette Wells, Christopher Parrish (MUN)
Collaborators: Beth Cleveland (USDA); Javier Santander (MUN); Adel El-Mowafi, Simon Wadsworth, Dominic Nanton, Renate Kvingedal (Cargill Canada)
Contact: mrise@mun.ca; richard_taylor@cargill.com

Dr. Chis Parrish and Jeanette Wells place a sample vial in a gas chromatograph’s autosampler, which is used to determine fatty acids in lipid extracts.
Photo: Genome Canada

Xi Xue working on quantitative PCRs with Drs. Richard Taylor and Matt Rise.
Photo: Genome Canada
Addressing challenges to Sablefish culture
The Sablefish (Anoplopoma fiimbria), also known as Black Cod, is a deep-water species that is widely distributed along the continental shelf of the Eastern and Western North Pacific. Sablefish has traditionally been supplied to the market by commercial fishers, but holds great promise as an aquaculture species on Canada’s West coast. For example, its market price is approximately twice that of salmon, and Golden Eagle Sable Fish is currently rearing Sablefish in British Columbia, and aims to reach an annual production of 500,000 juveniles and 1000+ tonnes of production in the next few years.
As with any new (alternative) aquaculture species, husbandry and management procedures developed for other fish species will have to be modified for Sablefish culture, and information on this species’ physiology, stress and disease resistance, and environmental tolerances must be gathered. Research conducted to date has determined the acute upper temperature and hypoxia tolerance of both adults and juveniles, and provided comprehensive information on this species’ stress and metabolic physiology. We are now developing genomic (i.e., molecular probes) and antibody-based tools (i.e., ELISAs) so that the immune function of this species can be studied, and future work on the efficacy of vaccines against furunculosis (caused by the pathogen Aeromonas salmonicida) is in the planning stages.
This research will provide key information on Sablefish health and welfare, and support the growth of this industry.
Date: Sep. 2016 – Ongoing
Funded by: Ocean Frontier Institute – Canada First Excellence Research Fund
Co-Funded by: Memorial University
Project Leader: Kurt Gamperl (MUN)
Project Team: Javier Santander, Robin Leeuwis, Fabio Zanuzzo, Rebeccah Sandrelli, Gord Nash (MUN)
Collaborators: Jessi Rix (Somru BioSciences); Stewart Johnson (DFO); Matt Rise (MUN); Tom Sorby, Briony Campbell (Golden Eagle Sable Fish Inc.); Rick Goetz (NOAA)
Contact: kgamperl@mun.ca

Graduate (M.Sc.) student Robin Leeuwis performing surgery on a Sablefish.
Photo: Robin Leeuwis (MUN)
Validation of blue diatom pigment production for industrial needs
Thanks to the development of a new mass production technique at the ISMER-UQAR Pointe-au-Père Aquaculture Station, we have developed a production method highlighting a benthic microalga that produces the blue pigment, marennine. We have demonstrated in the laboratory that this pigment protects bivalve larvae, in addition to being an antioxidant, antiproliferative and antivirulent agent; all properties that were validated in vitro.
This pigment offers an interesting natural alternative to conventional antibiotics in aquaculture, which is a growing market in Canada and around the world. Aquaculture has been the fastest growing food production sector in the last 50 years, with aquaculture products accounting for about 50% of the fish, shellfish and echinoderms consumed each year, worldwide. Production is a standardized market for almost 25% of the world aquaculture production. There is a real need for new molecules that have antibacterial and antioxidant abilities. These molecules will certainly be found within natural resources, since natural products are the best source of novelty and their molecular complexity makes their artificial synthesis difficult. Thus, the objective of this project is to develop a system of continuous mass production of this pigment in order to transfer it to one of our industrial partners, optimize its harvest and its purification in order to limit losses, evaluate its cost of production and significantly advance its characterization in order to facilitate the exploration of cosmetic markets by our second industrial partner.
This project will develop a system of marennine production, and will also highlight the potential colouring, antioxidant and antibiotic properties of the molecule that could contribute to many aquaculture applications, as well as in the food industry or the pharmaceutical field.
Date: Jun. 2018 – Jun. 2020
Funded by: Natural Sciences and Engineering Research Council (NSERC)
Project Leader: Réjean Tremblay (ISMER)
Project Team: William Belanger (ISMER/UQAR); Martin Forêt (ISMER)
Collaborators: Richard Saint-Louis, Jean-Sébastien Deschênes (UQAR)
Contact: Rejean_Tremblay@uqar.ca

One hundred-litre photobioreactors used for the production of the pigment marennine, produced by the benthic diatom Haslea ostrearia.
Photo: Martin Forêt (ISMER)
Comparison of three systems for removing carbon dioxide in recirculating salmon smolt hatcheries
The main objective of this project was to determine the most cost-effective method to maintain the CO2 concentration below 15 mg/L in recirculating aquaculture systems (RAS). Models were developed to predict CO2 removal across three degassing systems: degassing tower, moving bed biofilter and side-tank degasser. Total system balances were also developed to predict steady state CO2, bicarbonate (HCO3-) and TAN concentrations in recirculation systems. The amount of caustic soda required to maintain alkalinity at desired conditions is also predicted by the steady state HCO3- model. All models were successfully validated with experimental data.
The moving bed biofilter and degassing towers studied were found to have a single pass removal efficiency of 35%. The removal efficiency of the two-cell moving bed biofilter takes into account the CO2 produced in the biofilter due to the nitrification process. The side-tank degassers had a lower removal efficiency, 20%; however, they removed the most CO2 per unit energy input (77 mg CO2/kJ). The CO2 mass transfer coefficient was found to be 0.15 min-1 for the moving bed biofilter, 0.6 min-1 for the degassing towers and 1.4 min-1 for the side-tank degassers. The most cost-effective degassing system was found to be a moving bed biofilter with an additional aerated cell not containing media.
The results of this research project can be used in the design of degassing systems for RAS.
Date: Sep. 2017 – Jun. 2018
Funded by: MITACS Accelerate
Co-Funded by: Cooke Aquaculture Inc.
Project Leader: Michel Couturier (UNB)
Project Team: Sarah Preston (UNB)
Collaborators: Cooke Aquaculture Inc.
Contact: cout@unb.ca; sarah.preston@unb.ca
Fish farm site-to-site connectivity using GPS tracked surface drifters and FVCOM-based particle tracking model
The three year cycle, single year class Aquaculture Bay Management Areas (ABMAs) were implemented in Southwest New Brunswick in 2006 as part of a multi-faceted effort to manage disease within fish farms and reduce the potential for the spread of disease between farms within geographic areas. The boundaries of the ABMAs were chosen such that the estimated exchange of waterborne pathogens between ABMAs on a tidal time scale (~12.5 h) was minimized. Although the ABMA approach to disease management seems to have been successful and included more than estimates of water exchange, the original ABMA structure imposed some operational (wharf usage, equipment movement restrictions, etc.) and socio-economic consequences.
Both the aquaculture industry and the Government of New Brunswick desire a better understanding of the potential for water exchange, and the associated exchange of pathogens, between the farm sites within the existing ABMAs so the implications of altering existing ABMA boundaries can be better assessed. This project therefore aims to replace the original barotropic hydrodynamic model with a new regional baroclinic oceanographic model (FVCOM), new trajectories of GPS tracked surface drifters and new time series of water currents. This information will be combined with existing information to calibrate and validate the new model implementation and to update estimates of water exchange pathways and rates between the fish farms and farming areas within the Southwest New Brunswick region of the Bay of Fundy.
The results will contribute to an updated understanding of the potential exchange pathways between marine finfish farms in Southwest New Brunswick. In turn, this will contribute to the identification and consideration of options that offer more socio-economic flexibility as well as stability, to the industry and employees of the area, including the potential for more geographically consistent and stable employment without increasing the estimated potential for exchange of water borne pathogens between farm areas, (i.e., between ABMAs).
Date: Apr. 2016 – Mar. 2019
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Atlantic Canada Fish Farmers Association (ACFFA)
Project Leader: Fred Page (DFO)
Project Team: Sarah Scouten, Frederick (Jack) Fife, Emily Nelson, Mike Head, Peter Kraska, Ashley Holmes, Sean Corrigan, Randy Losier (retired), Claire Mussells, Graham Bartlett, Daryl Haughn, Rebecca Goreham, Susan Haigh, Mitchel O’Flaherty-Sproul (DFO); Mike Beattie (retired), Thomas Ogilvy (retired), Pat Mowatt, Gage Monteith, Joel Richardson, Bruce Thorpe (deceased), Sandi McGeachy (NBDAAF); Dwayne Richardson (Cooke Aquaculture); Al Pineau (Northern/Marine Harvest)
Collaborators: Sue Farquharson, Betty House (ACFFA)
Contact: Fred.Page@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/16-1-M-03-eng.html

GPS-tracked surface drifters.
Photo: Sarah Scouten (DFO)
A sustainable blue economy: More opportunities from salmon waste resources
Sustainable blue economy refers to the production of multiple co-products from marine waste biomass. From the Atlantic Salmon processing industry, 40–50% of the fish body is processed into by-products (head, gut and frame). These by-products are a rich source of valuable components such as collagen, protein, bioactive peptides, oil and omega-3 fatty acids.
This project is investigating the effective extraction of fish oil, protein and omega-3 fatty acids with pharmaceutical and nutraceutical potential. In order to maximize the yield and improve the quality of the products, the extraction procedure will be optimized through the screening of enzymes and antioxidants used, and the adjustment of the amount of water added, and the reaction temperature and reaction time. Characterization of the oil and protein will be performed to determine its quality and the omega-3 fatty acid profile will be analyzed.
Meanwhile, the project also explores the efficient handling, pre-treatment and storage conditions for salmon by-products to obtain pharmaceutical and nutraceutical value-added products. It is essential to optimize proper handling, pre-treatment and storage conditions to preserve the freshness of the materials for a longer time without additional costs to the existing industrial chain. Different pre-treatment techniques such as antioxidant addition, freeze drying and high pressure processing will be investigated. The effect will be determined from both the status of the by-products (e.g., colour, smell and microbial analysis) and the yield and quality of the extracted oil (e.g., peroxide value and lipid profile).
The research will provide essential laboratory and scale-up data about the efficient utilization of salmon waste for value-added products. The results will be used to carry out techno-economic feasibility studies and give us the opportunity to invest in future technologies to better serve the regional and economic development of the province and motivate the government to invest in this innovative, larger-scale research initiative for subsequent commercial applications. It will create new markets for salmon waste resources and improve the financial stability, in addition to the creation of new job opportunities in rural communities.
Date: Sep. 2018 – Sep. 2020
Funded by: Ocean Frontier Institute
Co-Funded by: Regional Aquaculture Centre
Project Leader: Deepika Dave (CASD)
Project Team: Yi Liu, Vegneshwaran V. Ramakrishnan, Isabel Cuenca, Wade Murphy (CASD)
Contact: Deepika.Dave@mi.mun.ca
Development of environmental DNA (eDNA)-based biosurveillance for aquatic invasive species (AIS) to inform management and policy decision-making associated with shellfish aquaculture movements
Shellfish movements associated with aquaculture activities are vectors for AIS introduction and spread. Informed decision-making around shellfish movements is an important management activity to mitigate risk of invasions in geographic areas in BC that currently lack AIS. DNA metabarcoding of complex (i.e., mixed-species or eDNA) samples generates millions of sequence reads from the majority of species present within the sample simultaneously and with high sensitivity.
The goal of this project is to develop an optimized and validated eDNA-based metabarcoding tool for AIS biosurveillance to aid decision-making around aquaculture shellfish movements in BC. To date we have developed eight DNA metabarcoding markers targeting high priority AIS in BC. We validated the tool in a pilot study which identified almost all known high priority AIS in the area in environmental and mixed-species (zooplankton) samples.
The tool is now being applied to the highly invasive European green crab on the west coast of Vancouver Island. The objective is to evaluate the relationship between DNA signal strength with crab density from catch data using metabarcoding and quantitative DNA assay approaches. To date we have determined that the quantitative assay can identify the presence of green crab at high density sites. The next step is to compare the quantitative assay with metabarcoding results to determine the tool’s suitability for green crab density assessment. Coastal sites throughout the Georgia Strait are also being studied to identify the first regional ’AIS profiles’ and compare the new biosurveillance tool with standard AIS survey techniques.
The validated metabarcoding tool will increase our capacity for early AIS detection and first response under a framework for making informed decisions about shellfish movement patterns around BC. This enhances our knowledge of AIS distribution throughout the province and allows management to define larger scale geographic units for shellfish movement purposes.
Date: Apr. 2016 – Mar. 2019
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Cathryn Abbott (DFO)
Project Team: Kristen Marie Westfall, Tom Therriault, Kristi Miller-Saunders, Kara Aschenbrenner, Geoff Lowe, Scott Gilmore (DFO); Melania Cristescu (McGill U); Guang Zhang (McGill U, DFO)
Contact: Cathryn.Abbott@dfo-mpo.gc.ca
Website: https://dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2016-P-03-eng.html

Collecting plankton samples for eDNA analysis using bongo nets.
Photo: Cathryn Abbott (DFO)
The validation of an FVCOM hydrodynamic model to support aquaculture on the west coast of Vancouver Island.
The West coast of Vancouver Island is complex oceanographically, geographically, biologically and meteorologically. It supports a vibrant fish farming industry. Previous work in other aquaculture regions (e.g., the Broughton Archipelago and the Discovery Islands) has demonstrated the advantage of having a hydrodynamic model that can provide information on current flow, temperature and salinity in three dimensions.
This project will examine and quantify uncertainties in the Finite-Volume, primitive equation Community Ocean Model (FVCOM) developed for the West coast under a previous ACRDP project (15-1-P-03). The model has been shown to be well suited for simulating the circulation and ecosystem dynamics, particularly for regions characterized by irregular, complex coastlines, islands, inlets, creeks, and inter-tidal zones. The research will seek to validate the model through identifying the degree of confidence that can be placed in the model results, and seek to optimize the model for applications in support of aquaculture.
Using the FVCOM output as input to particle tracking models already developed at the Institute of Ocean Sciences (IOS), the dispersion of virtual particles will be modeled and compared to the tracks of surface drifters released at key locations in the model domain. The results of this model will map where virtual particles are advected. Running the model with freshwater and wind forcing, in addition to the tides, the results will be compared to the acoustic Doppler current profiler (ADCP) currents and the water properties data collected at the fish farms. The comparison between model results and observed conditions will be used to assess how well the model simulates these combined processes.
The project will provide a FVCOM model for West coast aquaculture that can generate three dimensional fields of temperature, salinity and current within confidence limits determined by a clear validation process. This hydrodynamic model provides the ability to identify how water circulation transports virtual particles (such as sea lice or viruses). This information can be applied to farm site selection, production limits and contingency planning of waterborne threats to fish farms.
Date: May 2017 – Apr. 2019
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cermaq Canada Ltd.; Grieg Seafood BC Ltd.
Project Leader: Peter Chandler (DFO)
Project Team: Mike Foreman, Glenn Cooper, David Spear (DFO); Bogdan Vornicu (Grieg Seafood BC Ltd.)
Collaborators: Barry Milligan, Eric Jensen (Cermaq Canada Ltd.); Tim Hewison (Grieg Seafood BC Ltd.)
Contact: Peter.Chandler@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-P-06-eng.html

Davis weather station installed at fish farm in Clayoquot Sound providing real-time data via internet. Wind and solar radiation data are used as input to the FVCOM hydrodynamic model of the West coast of Vancouver Island.
Photo: Glenn Cooper (DFO)
Evaluation of the development of gametophytes in the sugar kelp, Saccharina latissima (order laminariales): Effect of the substrate, current and seeding technique
Kelp can develop into dense underwater forests that make a significant contribution to the productivity, biodiversity and vitality of coastal animal communities. However, in some parts of the world, there is a downward trend in their numbers, possibly related to climate change and human disturbance. This project, led by Merinov, proposes the implementation of an artificial reef seeding pilot project based on young seedling production and/or gametophyte/sporophyte production of macroalgae species native to the coastal areas of Quebec.
The participation of the Montreal Biodome will allow us to study, under controlled conditions, the effects and interactions of various environmental factors (current velocity, temperature, light, turbidity, etc.) on the growth of sporophytes for multiple kelp species indigenous to the Saint-Laurent (e.g., Saccharina latissima, Alaria esculenta and Saccorhiza dermatodea). This will allow us to optimize the techniques based on environmental conditions in order to ensure the success of seeding efforts in a natural environment.
The summer 2018 growth trials assessed the average growth and developmental intensity of young S. latissima seedlings based on current velocity, substrate type and gametophyte spray pattern. The data are being analyzed and the results of the study will provide recommendations as to the techniques to be used to promote the growth of these species, specific to the environmental context.
Date: Jan. 2018 – Mar. 2020
Funded by: DFO – Coastal Restoration Fund (DFO – CRF)
Co-Funded by: Montreal Biodome
Project Leader: Nathalie R. Le François (Montreal Biodome); Nicolas Lemaire (Merinov)
Project Team: Marie-Pomme Poissant, Jasmine Prégent, Jean-Christophe Boussin (Montreal Biodome); Mathilde Lemoine, Grégoire Cholat-Namy (Merinov)
Contact: NLe_Francois@ville.montreal.qc.ca
Advanced developments in DFO's FVCOM models for the discovery islands and Broughton Archipelago
The complex coastal waterways northeast of Vancouver Island support a vibrant fish farming industry. To ensure the sustainable management of the industry, understanding the regional hydrology is essential given the potential connectivity between some farms.
Previous work developed hydrodynamic models with FVCOM for the Broughton Archipelago and the Discovery Islands. These models have demonstrated the benefits of being able to simulate with confidence current flows, water temperature and salinity in three dimensions. FVCOM has been proven to provide this information and address area-based management issues, such as particle tracking, virus dispersion and sea lice infectivity trends.
This current project, PARR-2018-P-05, will advance the overall capabilities of FVCOM. Firstly, the existing FVCOM model for the Broughton Archipelago will be reconfigured to provide higher resolution output in the area around Port Hardy. Secondly, a biogeochemical module will be implemented within FVCOM and applied to the existing Discovery Islands FVCOM hydrodynamic model. With this biogeochemical-hydrodynamic coupled model, we will examine oxygen dynamics and the processes that control oxygen concentrations in a region with finfish aquaculture. Both of these FVCOM developments are logical extensions of the work already completed and will improve the quality and range of coverage available for area-based aquaculture management decisions.
Date: Apr. 2018 – Mar. 2020
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Leader: Laura Bianucci (DFO)
Project Team: Mike Foreman, Peter Chandler, Di Wan, Glenn Cooper (DFO)
Collaborators: Pramod Thupaki, Jen Jackson (Hakai Institute); Tarang Khangaonkar (PNNL); Susan Allen (UBC)
Contact: Laura.Bianucci@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2018-p-05-eng.html
Improving fertilization success of Arctic Charr
Wild Arctic Charr populations have a history of overharvest and remaining small-scale fisheries are under tight government regulation. Given a high market demand, a high market price, and the suitability of this species for production under high densities, Arctic Charr seems an excellent choice for the development of a sustainable land-based aquaculture industry. However, development of Arctic Charr as a viable aquaculture species has been limited by poor and variable fertilization success and egg survival rates. This project is tracking egg development, survival, hatching success and early performance of the fry during the first four to six months after initial feeding. The primary goal of the research is to improve the fertilization success, hatchability, and early survival of cultured Arctic Charr by optimizing the diet and defining rearing and spawning protocols.
The objectives of this project are to: 1) Formulate and test a diet for Arctic Charr; 2) Optimize gamete collection, storage, and fertilization protocols; and 3) Assess impacts of optimized diet and fertilization on early-rearing performance. To date, we developed a diet containing black soldier fly larvae (BSF). We replaced 20% of the fishmeal in a standard diet with BSF. Results indicated that BSF can be used to replace up to 20% of the protein source in a standard Arctic Charr diet without significantly affecting growth performance or feed efficiency. There was also improved survival in the first two months of the fry stage for fish fed the diet with BSF.
These results will be relevant for further growth and development of Arctic Charr aquaculture. Increased fertilization rates would provide an immense savings on rearing space, water use, electricity, and waste output. BSF appears to be a viable source of protein and/or fat in diets for Arctic Charr, and may provide a health benefit during early rearing.
Date: Apr. 2017 – Mar. 2019
Funded by: DFO–Aquaculture Collaborative Research and Development Program (DFO–ACRDP)
Co-Funded by: Taplow Ventures Inc.
Project Leader: Ian Forster, Robert Devlin (DFO)
Project Team: Leigh Gaffney; Brad Hicks (Taplow Ventures Inc.); Katarina H. Doughty (DFO)
Collaborators: Wendy Vandersteen (Miracle Springs Inc.)
Contact: Ian.Forster@dfo-mpo.gc.ca; Robert.Devlin@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/17-1-P-04-eng.html
Development of the Baynes Sound environmental intelligence collaboration (BaSEIC)
Baynes Sound, in the northern Salish Sea, is the epicenter of the BC shellfish aquaculture industry, supplying approximately 50% of production province-wide. Numerous studies have shown the vulnerability of commercial shellfish species to ocean acidification (OA), specifically through changes in seawater chemistry that affect calcium carbonate mineral saturation. A lack of environmental data detailing the biogeochemical conditions in Baynes Sound, as well as current issues with shellfish production and mortality, has led to growing concern regarding the potential impacts of OA. In aid of this problem, the BC Shellfish Growers Association (BCSGA) and local shellfish growers partnered with the Hakai Institute to form the Baynes Sound Environmental Intelligence Collaboration (BaSEIC), with support from the Province of British Columbia and the Tula Foundation.
The goal of this research initiative is to develop our understanding of current biogeochemical patterns in Baynes Sound using a framework that provides publicly accessible real-time data, as well as additional information through the collection of discrete samples. A measurement system was installed at Fanny Bay Oysters to provide high-frequency CO2 measurements made on seawater drawn from three to six meters depth adjacent to the facility, and these data are available live on a web portal (Global Ocean Acidification Observing Network data portal: http://www.ipacoa.org/Explorer?action=oiw:fixed_platform:FBO_Fannybay1). This continuous record, along with seasonally-resolved and spatially-distributed discrete seawater samples collected by shellfish growers, provide a solid baseline for OA conditions in Baynes Sound.
This initiative produces information essential for the BC shellfish aquaculture industry. The growing dataset reveals dynamic CO2 system patterns in Baynes Sound. The baseline information generated by this research is also a foundation for further areas of study such as phytoplankton, bacterial, and viral ecology, which may further shed light on potential drivers of shellfish mortality.
Date: JAN. 2017 – ONGOING
Funded by: Hakai Institute
Co-funded by: BC Shellfish Grower’s Association (BCSGA); BC Ministry of Agriculture; Tula Foundation
Project leader: Wiley Evans (Hakai Institute)
Project team: Katie Pocock, Alex Hare, Carrie Weekes (Hakai Institute)
Collaborators: BC Shellfish Grower’s Association; Fanny Bay Oyster Company; Mac’s Oysters; Deep Bay Marine Field Station (VIU)
Contact: wiley.evans@hakai.org

The BCSGA Burke-O-Lator (BoL) pCO2/TCO2 analyzer, installed at Fanny Bay Oysters in Baynes Sound, tracks seawater CO2 content.
Photo: Wiley Evans (Hakai Institute)
The development of an FVCOM hydrodynamic model to support aquaculture on the west coast of Vancouver Island.
Finite Volume Coastal Ocean Model (FVCOM) was applied to model the hydrodynamics in the Broughton Archipelago and the Discovery Islands. Since FVCOM’s development process is complex, this research project used existing information and new data collected specifically for this project in order to further develop the model. Given the importance of fresh water forcing to local inlet conditions where aquaculture operations take place, additional attention was paid to river discharges.
The ultimate goal of the project was to apply accurate 3D information from the FVCOM to examine particle tracking, virus dispersion, and sea lice behaviour in the waters off the West coast of Vancouver Island in and around aquaculture sites.
The resulting model can simulate the features of large scale circulation, and shows agreement with data collected using moored instruments at key locations in the region. It provides the ability to identify how water circulation transports virtual particles (such as sea lice or viruses). This information can be applied to farm site selection, production limits and contingency planning of waterborne threats to fish farms. This model for the West coast of Vancouver Island is now being validated prior to its use for decision-making.
The results of this project will be important for the sustainable management of the aquaculture industry. The data will inform mitigation actions to well characterized risk. From an industry perspective, these results can inform best management practices in order to offset possible impacts.
Date: Jul. 2015 – Mar. 2017
Funded by: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP)
Co-Funded by: Cermaq Canada Ltd.; Grieg Seafood BC Ltd.
Project Leader: Peter Chandler (DFO)
Project Team: Mike Foreman, Darren Tuele, John Morrison, Pramod Thupaki, Di Wan, Ming Guo, Maxin Krassovski (DFO)
Collaborators: Barry Milligan (Cermaq Canada Ltd.); Tim Hewison (Grieg Seafood BC Ltd.)
Contact: Peter.Chandler@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/15-1-P-03-eng.html
Evaluation and comparison of Cranford & Wong and ISE methods for sediment sulfide and oxygen measures in aquaculture-related organically enriched sediments using manipulative and observational approaches
Newly developed analytical methods to measure total free sulphide and dissolved oxygen concentrations in sediments (metrics used to assess the oxic state of sediments) need to be evaluated more extensively in the field because these concentration gradients are used to predict benthic impacts from organic loading at aquaculture operations.
This project will use two sediment sources. The first is a mesocosm approach to generate a gradient of mussel biodeposition rates similar to those created by bivalve aquaculture. The second is at a commercially-operated salmon farm, sampled along a presumed organic enrichment gradient during maximum fish production levels. We will compare the total sulphide and dissolved oxygen concentrations using the new methods with those measured using the current (ISE) methods and see how both of these metrics are related to sediment benthic community structure. In addition, nutrient fluxes from sediment cores from the mesocosms will be measured to better understand the relationship between organic loading and sediment processes like respiration and nutrient recycling.
This project builds upon ongoing projects funded by DFO’s Program for Aquaculture Regulatory Research (PARR-2016-Q-01 and PARR-2016-Q-16) and takes advantage of the specialized staffing (i.e., dive team) working in the region. These results will improve the accuracy of the definition of the oxic state of sediments and better inform aquaculture management when assessing aquaculture operations today.
Date: Apr. 2017 – Mar. 2018
Funded by: DFO – Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Chris Mckindsey (DFO)
Contact: Chris.Mckindsey@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-q-09-eng.html
Operational management of sustainable fish farming
Most academic research in aquaculture sustainability has been carried out at the farm-scale, while an understanding of ecosystem-scale measures of sustainability is still in its infancy. The need for an ecosystem approach to aquaculture (EAA) is made apparent by the fact that regulatory processes are currently directed at localized benthic effects at the farm-scale, preventing the integration of ecosystem-scale sustainability research into aquaculture management. The transition from a local (farm-scale) to ecosystem (bay-scale) approach to regulation has not been realized because mechanisms for its implementation are poorly developed. Building on our extensive record in models and field studies of aquaculture, the proposed research will focus on these key gaps in aquaculture sustainability through the following objectives:
- Operationalize ecosystem science into a decision support and management system for continued improvement of husbandry and future growth for salmon farming;
- Execute the practice of an ecosystem approach to aquaculture (EAA) as a successful model for sustainable fish farming; and
- Lead the development and implementation of international best practices for compliance with both government regulations and third-party standards.
These objectives will be undertaken through five modules that combine modelling, field work and analysis of aquaculture regulations and certifications: (1) Informing risk assessment with operational tools of coastal connectivity; (2) FINS (Fish-farm Interactions with Natural Systems): Decision support software for aquaculture management using benthic health as a benchmark; (3) Field implementation of an ecosystem approach in aquaculture regulation using cost-effective monitoring; (4) Global integration of environmental standards; and (5) Eco-certification: bringing clarity to third party standards.
The strong focus of the proposed research on operational tools and technological applications is designed to directly benefit industry and government regulators. The objective analysis of regulations and certification bodies will also benefit Canadians, potentially increasing the social license for Canadian salmon aquaculture.
Date: Jan. 2018 – Dec. 2020
Funded by: Natural Sciences and Engineering Research Council (NSERC) – Collaborative Research and Development Grants
Co-Funded by: Cooke Aquaculture Inc.
Project Leader: Jon Grant (Dalhousie U)
Project Team: Ramón Filgueira (Dalhousie U)
Collaborators: Longline Environment Ltd.
Contact: Jon.Grant@dal.ca
Rapid and sensitive eDNA methods for early detection and mitigation of aquatic invasive species (AIS) and monitoring of aquatic species at risk (ASAR)
Tunicates are especially detrimental to mussel aquaculture in PEI, and create an important economic burden to producers. This work will provide information on the presence and distribution (e.g., distribution maps) of tunicates and other known aquatic invasive species (AIS) as well as the Brook Floater (Alasmidonta varicose), a species of special concern under COSEWIC and SARA. The information can then guide decision on mitigation strategies and conservation efforts.
The detection of species using environmental DNA (eDNA) in water samples is a promising approach in support of traditional field surveys for the management and conservation of aquatic species. The method is also gaining popularity as an early detection tool. While traditional monitoring techniques can be limited in morphologically identifying cryptic species or larval life stages, eDNA detection can help resolve these problems, as it is sensitive, rapid and specific. As such, eDNA testing has great potential in aquatic ecosystems to monitor AIS that affect aquaculture (e.g., tunicates) as well as rare and elusive species (e.g., the Brook Floater).
The goal of this project is to develop, evaluate and optimize eDNA based tests for AIS (nine established and ±15 potential), as well as a species of special concern, the Brook Floater. Species-specific molecular tests (qPCR) will be designed and evaluated for each species using the widely used cytochrome c oxidase 1 (COI) DNA barcode. Barcoded sequences will be uploaded into the Barcode of Life website (BOLD) and vouchers will be sent to museum for future reference. eDNA capture methods (e.g., water sampling, collector plates) will also be evaluated on a select list of species (four to five) to optimize the approach in terms of sensitivity, feasibility and costs. Finally, the suitability of the tests will be evaluated on field samples and the results compared to other traditional methods.
This study will provide proof-of-concept for the suitability of eDNA testing to detect and estimate the abundance of aquatic species.
Date: Apr. 2017 – Mar. 2019
Funded by: DFO - Genomics Research and Development Initiative (DFO – GRDI)
Project Leader: Francis Leblanc (DFO)
Project Team: Valérie Belliveau, Jean-René Arseneau, Nellie Gagné, Renée Bernier, Chantal Coomber, Erica Watson, Nathalie Simard, Cynthia Mckenzie (DFO)
Contact: Francis.LeBlanc@dfo-mpo.gc.ca

Francis Leblanc sampling water for eDNA detection of aquatic species.
Photo: DFO
What is the overall effect of shellfish aquaculture on eelgrass, an ecologically significant species?
Eelgrass (Zostera marina) was designated an ecologically significant species because it provides numerous ecological functions, including habitat for fish and their prey. The interactions between oyster aquaculture and eelgrass beds will be analyzed in this project, which aims to assess the net effects of oyster farming on CRA fisheries and eelgrass habitat. This project will synthesize the results from past research projects funded by DFO’s Program for Aquaculture Regulatory Research (i.e., PARR-2011-G-19, PARR-2012-G-01, and PARR-2014-G-10) and the scientific literature to produce a review of the scale and scope of interactions between shellfish aquaculture and eelgrass. At the small scale, bare patches of eelgrass occurred under oyster farms and research revealed that shading from oyster aquaculture structures were the primary stressor. As research expanded to assess bay-scale effects, the hypothesis that oyster farming can increase eelgrass productivity due to increased water clarity was explored. Impacts on eelgrass beds may reduce some CRA fisheries’ productivity with habitat loss; however, the ability of aquaculture structures to create new fish habitat may offset this loss of CRA fisheries. This project will review the influence of oyster aquaculture structures on eelgrass, the creation of fish habitat by aquaculture structures, and the overall consequences on fish habitat change.
Date: Apr. 2017 – Ongoing
Funded by: DFO–Program for Aquaculture Regulatory Research (DFO–PARR)
Project Leader: Jeffrey Barrell (DFO)
Project Team: Remi Sonier, Marc Ouellette (DFO)
Contact: Jeffrey.Barrell@dfo-mpo.gc.ca
Website: http://www.dfo-mpo.gc.ca/aquaculture/rp-pr/parr-prra/projects-projets/2017-g-06-eng.html
Social licence and planning in coastal communities
This module of the Ocean Frontier Institute aims to develop innovative mechanisms for bridging the gap that currently exists in understanding Social Licence to Operate (SLO) for aquaculture by focusing on three normative SLO components (legitimacy, credibility, and trust) and four levels of social licence (withdrawal, acceptance, approval, and identification with the project). SLO as a concept was first articulated in the mining sector, but it has quickly spread to other resource intensive sectors including aquaculture.
The term SLO can be defined as a community’s understanding and experience of the benefits and impacts of resource development. It is widely acknowledged that social licence is an ongoing practice that changes over time. SLO as a process is often linked to, and can inform, other legal and regulatory arrangements including impact assessments (social and environmental), corporate social responsibility (CSR), sustainability certification systems, and consumer advice programmes. Although social licence is frequently cited as a key goal in Canadian provincial and national policy documents on aquaculture, and is a strong feature of international concerns around aquaculture, it remains poorly defined in this key sector of the marine economy.
The goal of Module M is to advance knowledge on social licence in aquaculture by establishing the expectations and roles of stakeholders involved in achieving sustainable aquaculture, and by developing and implementing tools to optimize and translate knowledge so that the full suite of factors affecting SLO can be better understood and outcomes predicted based on this increased understanding.
This research will produce regionally vital information for a rapidly developing aquaculture sector and a globally exportable integrated framework for SLO in aquaculture, filling a significant knowledge gap in the sector as evidenced in Canada, Europe and other leading marine aquaculture producing areas in the world. Its innovation lies in the integration and diversity of new knowledge affecting SLO, co-created with key stakeholders and leading to an enhanced understanding of social licence, regulation and resource sharing.
Date: Apr. 2017 – Mar. 2022
Funded by: Ocean Frontier Institute
Project Leader: Lucia Fanning (Dalhousie U); Charles Mather (MUN)
Project Team: Dean Bavington, Reade Davis, Faisal Khan, Joel Finnis, Paul Foley, Zhiwei Gao, Max Liboiron, Lorenzo Moro, Barbara Neis, Nicole Power (MUN); Ramon Filgueira, Jon Grant, Bertrum MacDonald, Patricia Manuel, Ian Stewart (Dalhousie U); Gesche Krause, (AWI, WGSEDA)
Collaborators: NAIA; SINTEFT; DFO; ASF; AWI; FFAW; IMR; NL-FFSA
Contact: Lucia.Fanning@dal.ca; cmather@mun.ca
Website: https://oceanfrontierinstitute.com/research/social-licence-and-planning-in-coastal-communities; soon to be launched: www.coastalfutures.ca
Glossary
- Term
- Definition
- AAFC
- Agriculture and Agri-Food Canada
- AANS
- Aquaculture Association of Nova Scotia
- AARS
- Alma Aquaculture Research Station (U Guelph)
- ABMA
- Aquaculture Bay Management Area
- ACFFA
- Atlantic Canada Fish Farmers Association
- AChE
- Acetylcholinesterase
- ACRDP
- Aquaculture Collaborative Research and Development Program
- ACOA
- Atlantic Canada Opportunities Agency
- ADCP
- Acoustic Doppler Current Profiler
- AEIP
- Aquaculture Ecosystems Interactions Program
- AGD
- Amoebic gill disease
- AHC
- Animal Health Centre (BC)
- AIF
- Atlantic Innovation Fund
- AIS
- Aquatic invasive species
- ASF
- Atlantic Salmon Federation
- AVC
- Atlantic Veterinary College
- AVC
- Atlantic Veterinary College (UPEI)
- AWI
- Alfred-Wegener Institute
- BaSEIC
- Baynes Sound Environmental Intelligence Collaboration
- BC
- British Columbia
- BCSFA
- BC Salmon Farmers Association
- BCSGA
- BC Shellfish Grower’s Association
- BKD
- Bacterial Kidney Disease
- BOD
- Biochemical Oxygen Demand / Biochemical Oxygen Demanding
- BOLD
- Barcode of Life Data System
- BSF
- Black soldier fly
- C
- Carbon
- C&A
- Central & Arctic Region, Fisheries and Oceans Canada
- CAAHRD
- Centre for Aquatic Animal Health Research and Diagnostics (DFO)
- CAHS
- Centre for Aquatic Health Sciences (BC)
- CAIR
- Centre d'appui à l'innovation par la recherche (UQAR)
- CARTI
- Centre for Applied Research, Technology and Innovation (NIC)
- CASD
- Centre for Aquaculture and Seafood Development (Marine Institute)
- CATC
- Center for Aquaculture Technologies Canada
- CBU
- Cape Breton University
- CCGS
- Canadian Coast Guard Ship
- CCHSI
- Chopin Coastal Health Solutions Inc.
- CDRF
- Cold-Ocean Deep-Sea Research Facility (MUN)
- CFIA
- Canadian Food Inspection Agency
- CFS
- Canadian Federation of Students
- CFU
- Colony-forming unit
- CGQ
- Centre de géomatique du Quebéc
- CHS
- Chitin synthase
- CMA
- Cornmeal agar
- CO2
- Carbon dioxide
- COI
- Cytochrome C oxidase 1
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- CPUE
- Catch per unit effort
- CRA
- Commercial, recreational or Aboriginal fisheries
- CRC
- Canada Research Chair
- CRIUCPQ
- Quebec Heart and Lung Institute
- CroV
- Cafeteria roenbergensis virus
- CRS
- Coastal restoration fund
- CSAS
- Canadian Science Advisory Secretariat
- CSIC
- Spanish National Research Council
- CSP
- Chitin synthesis pathway
- CSR
- Corporate social responsibility
- CTD Station
- Instrument for measuring ocean conductivity, temperature, and depth
- Dalhousie U
- Dalhousie University
- DFARD
- Department of Fisheries, Aquaculture and Rural Development (PEI)
- DFO
- Fisheries and Oceans Canada
- DI
- Discovery Islands
- DNA
- Deoxyribonucleic acid
- DO/DO2
- Dissolved oxygen
- DTU
- Technical University of Denmark
- Duke U
- Duke University
- EAA
- Ecosystem approach to aquaculture
- EAF
- Embryo/alevin/fry
- ECCC
- Environment and Climate Change Canada
- eDNA
- Environmental DNA
- ELISA
- Enzyme-linked immunosorbent assay
- EMB
- Emamectin Benzoate
- EU
- European
- FAP
- Fonds d'Amorçage de Partenariat
- FASAM
- Agricultural Training for Food Security in Mali
- FCR
- Feed conversion rate
- FFAW-Unifor
- Food Fish and Allied Workers Union
- FINS
- Fish-farm interactions with natural systems
- FMM
- Flexibacter maritimus
- FRQNT
- Fonds de recherche du Québec – Nature et technologies
- FVCOM
- Finite Volume Coastal Ocean Model
- g
- Gram
- GAIN
- Green Aquaculture Intensification in Europe
- GC
- Gas chromatograph
- GFAT
- Glucosamine: fructose-6-phosphate aminotransferase
- GIS
- Geographic Information System
- GRDI
- Genomics Research and Development Initiative (DFO)
- HCO3
- Bicarbonate
- HMSC
- Huntsman Marine Science Centre
- HSMI
- Heart and Skeletal Muscle Inflammation
- HPR0
- Low virulent infectious salmon anaemia virus
- ICES
- International Council for the Exploration of the Sea
- IFREMER
- French Research Institute for Exploration of the Sea
- IgM
- Immunoglobulin M
- IHNV
- Infectious hematopoietic necrosis virus
- IMR
- Institute of Marine Research, Norway
- IMTA
- Integrated Multi-Trophic Aquaculture
- IOS
- Institute of Ocean Sciences
- IP
- Intraperitoneal
- IPMC
- Integrated Pathogen Management of Co-infection
- IRAP
- Industrial Research Assistance Program (NRC)
- IRAP
- Integrated respiratory assessment paradigm
- IRCC
- Industrial Research Chairs for Colleges
- ISA
- Infectious salmon anemia
- ISAV
- Infectious salmon anemia virus
- ISE
- Ion selective electrode
- ISMER
- Institut des Sciences de la Mer de Rimouski (UQAR)
- ITS
- Internal transcribed spacer
- JBARB
- Joe Brown Aquatic Research Building
- k
- Kilobase
- KCS
- Kelly Cove Salmon Ltd.
- KJ
- Kilojoule
- LARSEM
- Laboratoire aquatique de recherche en sciences environnementales et médicales
- LC50
- Lethal concentration required to kill 50% of a trial group
- LL
- 24 hour light
- LOEC
- Lowest observable effect concentration
- m
- Meter
- MA
- Marine agar
- MACAS
- Ministerial Advisory Committee on Atlantic Salmon
- MAFI
- Magellan Aqua Farm Inc.
- MAPAQ
- Quebec Department of Agriculture, Fisheries and Food
- MB
- Manitoba
- Mc
- Myxobolus cerebralis
- MC
- Microcystin
- McGill U
- McGill University
- MDS
- Multidimensional scaling
- MÉES
- Québec Ministry of Higher Education and Training
- MERP
- Marine Environmental Research Program (BCSFA)
- MESI
- Ministère de l’Économie, de la Science et de l’Innovation
- MET
- Master of Educational Technology (UBC)
- mg
- Milligram
- ml
- Millimeter
- MMAFMA
- Mi’gmaq Maliseet Aboriginal Fisheries Management Association
- MMR
- Maximum metabolic rates
- Mount Allison U
- Mount Allison University
- MP
- Microplastic particles
- MSc
- Master of Science
- MSU
- Montana State University
- MSX
- Haplosporidium nelsoni
- MSX
- Multinucleate Sphere X
- MT
- Metric ton (one thousand kilograms)
- MUN
- Memorial University of Newfoundland
- NA
- North Atlantic
- NAIA
- Newfoundland Aquaculture Industry Association
- NB
- New Brunswick
- NBDAAF
- New Brunswick Department of Agriculture, Aquaculture and Fisheries
- NBIF
- New Brunswick Innovation Foundation
- NCAG
- National Contaminants Advisory Group
- NIC
- North Island College
- NINA
- Norwegian Institute for Nature Research
- N
- Nitrogen
- N2
- Dinitrogen
- NL
- Newfoundland and Labrador
- NL-FHSA
- Newfoundland Fish Harvesting Safety Association
- NMBU
- Norwegian University of Life Sciences
- nmol
- Nanomole
- NO2
- Nitrogen dioxide
- NO3
- Nitrate
- NH4
- Ammonia
- NOAA
- US National Oceanographic and Atmospheric Administration
- NOEC
- No observed effect concentration
- NPLD
- Net pen liver disease
- NRC
- National Research Council
- NRCAN
- Natural Resources Canada
- NS
- Nova Scotia
- NSDFA
- Nova Scotia Department of Fisheries and Aquaculture
- NSERC
- Natural Sciences and Engineering Research Council
- NTNU
- Norwegian University of Science and Technology
- NV
- Namao virus
- OA
- Ocean acidification
- OFI
- Ocean Frontier Institute
- OMAFRA
- Ontario Ministry of Agriculture, Food and Rural Affairs
- P
- Phosphorus
- PARR
- Program for Aquaculture Regulatory Research
- PART
- Programme d'aide à la recherche et au transfert (MÉES)
- PCA
- Principal Component Analysis
- pCO2
- Dissolved carbon dioxide
- PCR
- Polymerase Chain Reaction
- PEI
- Prince Edward Island
- PEIAA
- Prince Edward Island Aquaculture Alliance
- PGD
- Proliferative gill disease/disorder
- PGI
- Proliferative gill inflammation
- PHAC
- Public Health Agency of Canada
- PIT
- Passive integrated transponder
- PML
- Plymouth Marine Laboratory
- PNNL
- Pacific Northwest National Laboratory
- PRV
- Piscine orthoreovirus OR piscine reovirus
- ppm
- Parts-per-million
- PPSI
- Priorities and Partnership Fund Strategic Initiatives
- psi
- Pounds per square inch
- PVC
- Polyvinyl chloride
- Q2
- Test method using real time polymerase chain reaction
- QAIC
- Quebec’s Agrifood Innovation Centre
- qPCR
- Quantitative (Real-Time) Polymerase Chain Reaction
- RAQ
- Ressources Aquatiques Québec
- RAS
- Recirculating aquaculture systems
- RBC
- Red blood cell
- RLI
- The Rivers and Lochs Institute (Inverness College)
- RMR
- Routine metabolic rates
- RNA
- Ribonucleic Acid
- RNA-seq
- RNA sequencing; whole transcriptome shotgun sequencing
- RNAi
- RNA interference
- ROV
- Remotely Operated Vehicle
- RPC
- Research and Productivity Council (NB)
- RT-qPCR
- Quantitative reverse transcription PCR
- SABS
- St. Andrews Biological Station
- SARA
- Species At Risk Act
- SAV
- Salmon alphavirus
- SAV3
- Third subtype of salmonid alphavirus
- SDB
- Sabouraud Dextrose Broth
- SFU
- Simon Fraser University
- SGPV
- Salmon gill poxvirus
- SINTEF
- Applied research, technology and innovation research organisation (Norway)
- SLO
- Social licence to operate
- sNCLDV
- Sturgeon nucleo-cytoplasmic large DNA virus
- SNP
- Single Nucleotide Polymorphism
- SOG
- Strait of Georgia
- SRS
- Salmonid Rickettsial Septicaemia
- SVCV
- Spring Viremia of Carp Virus
- TAM
- Tumor-associated macrophage
- TAN
- Total ammonia nitrogen
- TFS
- Total free sulfides
- U
- University
- U Alabama
- University of Alabama
- U Alberta
- University of Alberta
- U Chile
- Universidad de Chile
- U Geneva
- University of Geneva
- U Guelph
- University of Guelph
- U Laval
- Université Laval
- U Moncton
- University of Moncton
- U Waterloo
- University of Waterloo
- U Windsor
- University of Windsor
- UBC
- Univeristy of British Columbia
- UCC
- University College Cork
- UdeM
- Université de Montréal
- UNB
- University of New Brunswick
- UNBF
- University of New Brunswick - Fredericton
- UNBSJ
- University of New Brunswick - Saint John
- UPEI
- Univeristy of Prince Edward Island
- UQAR
- University of Quebec - Rimouski
- UROC_Q
- Quebec Arctic Char Research Group
- USDA
- United States Department of Agriculture
- USGS
- United States Geological Survey
- UVic
- University of Victoria
- VHSV
- Viral hemorrhagic sepicemia virus+B248
- VIU
- Vancouver Island University
- WFRC
- Western Fisheries Research Center
- WGSEDA
- Working Group on Social and Economic Dimensions of Aquaculture
- WLU
- Wilfrid Laurier University
- YC
- Year class
- Date modified:



