Canadian Aquaculture R&D Review 2015
Table of Contents
Shellfish: Other
TRANSPORTING LIVE SEA SCALLOPS TO QUEBEC’S URBAN MARKETS
Sea Scallop (Placopecten magellanicus) growers in maritime Quebec have, for many years, sought to market their live product in the urban markets of Québec City and Montreal. Currently, Culti-Mer inc. in les Îles-de-la-Madeleine, sends live scallops by air for sale in these markets. Once they reach distributors, the scallops are kept in cold storage until they are sold. This luxury good is sold live for more freshness and is generally eaten whole. Unfortunately, the short shelf life of live scallops is a limiting factor, to say nothing of the fact that sending them to markets by air depends on often unpredictable weather. The goal is to extend the lifespan of the scallops once they hit the markets in large centres in a similar fashion to live lobster found in restaurants and fish markets. To solve this problem, in 2013–2014, Merinov tested two closed-circuit autonomous holding systems from Nova Scotia-based BioNovations. The performance of these systems was compared to the traditional method used by the company; transport and dry storage in a styrofoam container (cooler). The preliminary results showed that transport in a styrofoam container followed by re-immersion in water is the more promising. Culti-Mer would like Merinov to continue to work to ensure conservation of the organoleptic properties (the aspects of food as experienced by the senses, including taste, sight, smell, and touch) and the safety of the product in the container.
May 2013–Jul. 2014
Funded By: Ministère des Finances et de l’Économie du Québec (MFÉ); Fonds d’Amorçage de Partenariat (FAP) (UQAR, Merinov)
Project Lead: Lisandre Solomon (Merinov)
Project Team: Merinov
Collaborators: UQAR; Culti-Mer Inc.; Pec-Nord Inc.
Contact: Lisandre.Solomon@merinov.ca

Sea Scallops. Photo: Jacques Richard
YEAR-ROUND SPAWNING AND LARVAL REARING OF GEODUCK (PANOPEA GENEROSA) IN CLOSED CULTURE SYSTEM
This simple and inexpensive closed-culture system for geoduck spawning can be replicated as needed. The portability of the University of British Columbia’s (UBC) design of a seawater filtration system can be adopted to prevent microbial contamination during microalgae production and for inducing geoduck broodstock spawning. The developed protocol for inducing broodstock spawning will improve geoduck gamete production and simplify hatchery operations.
Geoduck is the most important commercial shellfish species in British Columbia. Unfortunately, commercial production is limited by the reliable supply of high quality hatchery-produced juveniles. Developing viable and sustainable culture protocols for this species will provide the industry with the needed seedstock to expand production. Benefits from expansion include new economic and employment opportunities for shellfish growers, seafood exporters, and First Nations communities plus a new source of sustainably produced seafood products. The UBC and British Columbia Pacific Oysters, Ltd. (BCPOL) have established a research collaboration to develop sustainable geoduck aquaculture production techniques through NSERC Engage and Engage Plus Grants. The preliminary research outcomes include: (1) design and construction of a prototype closed-culture system for controlled geoduck spawning; (2) fabrication of an elegant yet inexpensive seawater filtration system; (3) successful induction of geoduck broodstock spawning based on environmental manipulation among desiccation, temperature shifts, UV-filtered water stimulation, microalgal addition, and various combinations of the above; and (4) successful development of inexpensive system for efficient and continuous culture of microalgae for feeding geoduck seedstock.
Apr. 2014–Sep. 2014
Funded By: Natural Sciences and Engineering Research Council (NSERC) Engage Co-Funded By: BC Pacific Oyster Ltd. (BCPOL)
Project Lead: Andrew Riseman (UBC)
Project Team: Jesse Ronquillo (UBC)
Collaborators: Tony Farrell (UBC); John Zhang, Daniel McDermid (BCPOL)
Contact: Andrew.Riseman@ubc.ca

Spawning of Geoduck (Panopea generosa) in closed culture system. Photo: Jesse Ronquillo (UBC)
A MICROALGAL PIGMENT FOR STABILIZING HATCHERY-REARED SEA SCALLOP (PLACOPECTEN MAGELLANICUS) PRODUCTION
The results of this project provide a natural solution to the issue of massive mortality related to bacterial infections in hatcheries. Use of this technology may improve this industry’s yields and productivity.
Sea Scallop (Placopecten magellanicus) is a sensitive species and despite recent major developments, spat production is still unstable and primarily affected by the presence of opportunistic pathogenic bacteria of the genus Vibrio. These infections cause massive mortalities in tanks and several commercial hatcheries use antibiotics systematically for prevention. However, with the emergence of antibiotic resistant bacteria, this practice is now very controlled and difficult to apply. There is a need for new molecules that possess antibacterial activities and would potentially be meant solely for aquaculture. The micro-alga Haslea ostrearia, produced in large volumes at Université de Québec à Rimouski, produces such a molecule. The effectiveness of this pigment, called marennine, for controlling the virulence of the pathogenic bacterium Vibrio splendidus and improving the quality of a larval culture of Mytilus edulis has already been demonstrated. This project showed that marennine treatment resulted in improvement of the physiological condition and survival of a P. magellanicus flow-through larval culture and greatly reduced the virulence of V. splendidus in a mortality-causing condition.
Aug. 2014–Dec. 2014
Funded By: Natural Sciences and Engineering Research Council (NSERC) Co-Funded By: Ressources Aquatiques Québec (RAQ)
Project Lead: Réjean Tremblay (UQAR)
Project Team: François Turcotte, Jean-Sébastien Deschênes (UQAR)
Contact: Rejean_Tremblay@uqar.ca
EARLY DEVELOPMENTAL STAGES OF GEODUCK (PANOPEA GENEROSA)
BC Pacific Oysters Ltd. (BCPOL), a Canadian company with a 12.7 hectare grow-out aquaculture facility in Jervis Inlet, British Columbia, has established a partnership with the Faculty of Agriculture, Dalhousie University (formerly Nova Scotia Agricultural College) and the University of British Columbia for the development of a commercial hatchery and seedstock production of geoduck (Panopea generosa). The initial research project activities focused on documenting the early embryonic and post-embryonic stages of geoduck to develop a hatchery production protocol. Geoduck broodstock were spawned through natural means and the larvae were reared until early juvenile stage. Primary results showed that after 50 days of culture from spawning, the juveniles reached approximately 7 to 12 mm shell length by feeding on microalgae such as chlorophyte, baccilariophyte, chrysophyte, and prymnesiophyte rich in protein with high level of omega-3 fatty acids. Survival rate was more than 90%, and no disease was found based on juvenile geoduck pathogen analysis conducted at Pacific Biological Station of the Department of Fisheries and Oceans Canada (DFO). The preliminary research results were very promising for the development of a viable commercial geoduck hatchery production system.
The initial results demonstrate the feasibility of developing a viable commercial hatchery production of healthy and good quality geoduck seedstock in Canada to supply the needs of geoduck growers in British Columbia and the United States.
Apr. 2009–Sep. 2014
Funded By: BC Pacific Oyster Ltd. (BCPOL)
Project Lead: Jesse Ronquillo (UBC)
Project Team: Audrie-Jo McConkey (Dalhousie U)
Collaborators: Andrew Riseman, Tony Farrell (UBC); John Zhang, Daniel McDermid (BCPOL)
Contact: Jesse.Ronquillo@ubc.ca
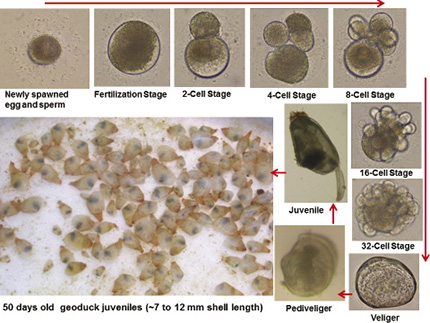
Embryonic and post-embryonic stages of geoduck (Panopea generosa) under controlled conditions. Photo: Jesse Ronquillo (UBC)
ARE SHELLFISH TRANSFERS A LIKELY VECTOR FOR AQUATIC INVASIVE SPECIES MOVEMENT FROM THE WEST TO THE EAST COAST OF VANCOUVER ISLAND?
The transfer of shellfish (including clams, oysters, and mussels) from areas infested with Non-Indigenous Species (NIS) can be a vector for both mobile and sessile NIS. There are currently no mitigation measures that are 100% effective in removing or destroying NIS on cultured shellfish. The objective of this study was to assess shellfish transfers by the shellfish industry and the Canadian Food Inspection Agency’s (CFIA) biotoxin monitoring program as potential vectors for the spread of aquatic invasive species (AIS), with particular focus on the European Green Crab, from the west to the east coast of Vancouver Island. The project quantified the potential risk of AIS introduction associated with current shellfish transfer protocols. Based on the results of the experimental and processor studies, the present conditions of licence do not eliminate the risk of transferring NIS. The gaps identified suggest that the intended reduction in propagule pressure is not being realized under current management/regulatory strategies. A conceptual framework was developed to identify potential control points where management intervention, such as the development of scientifically-based shellfish aquaculture management zones and the application of license conditions could be used to lower propagule pressure and hence invasion risk in British Columbia.
Apr. 2011–Mar. 2014
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Chris Pearce, Hannah Stewart (DFO)
Project Team: Tom Therriault, Graham Gillespie, Lyanne Burgoyne (DFO)
Collaborators: Gordy McLellan (Mac’s Oysters Ltd., BC); Deidre Kelly (CFIA)
Contact: Chris.Pearce@dfo-mpo.gc.ca; Hannah.Stewart@dfo-mpo.gc.ca
THE ECOLOGICAL EFFECTS OF CLAM HARVESTING BY MECHANICAL MEANS IN ST MARY’S BAY, NOVA SCOTIA
Traditional hand harvesting is not considered to be a sustainable practice for providing seed for the development of clam aquaculture in Nova Scotia for various reasons, including social and economic factors. The clam aquaculture industry has experienced major challenges in the recruitment and retention of clam diggers, as well as a lack of interest from the younger employable population, resulting in an aging employee-base. Additionally, traditional hand harvesting is very labour-intensive and involves the use of a clam hake, having tines that measure about 15 cm in length, to dig up and turn over the sediment. A mechanical clam harvester has been used in Washington and British Columbia. There is increased interest in utilizing a modified version of this harvester to compliment hand harvesting of quahogs (Mercenaria mercenaria) in St Mary’s Bay, Nova Scotia. This project will compare the ecological effect of traditional hand harvesting and a mechanical clam harvester. It will investigate the effects of each harvest technique on the ecological health and production of the area through the monitoring of the clam population, associated fauna and flora, and various physical and chemical parameters. Methods for reducing the ecological impact of harvesting, such as replanting pre-recruits on size-class plots and reducing repeated harvesting efforts will also be investigated.
Apr. 2012–Mar. 2015
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Innovative Fisheries Products
Project Lead: Thomas Landry (DFO)
Collaborators: Innovative Fisheries Products
Contact: Thomas.Landry@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
UNDERSTANDING THE DISTRIBUTION OF A NEMERTEAN PREDATOR, CEREBRATULUS LACTEUS, IN CLAM FLATS: IMPLICATIONS FOR CONTROL MEASURES
The results of this project will provide information to better understand the factors involved in the patchy distribution and abundance of Cerebratulus lacteus. This information will aid in the development of efficient management strategies to minimize the effect of this predator on clam populations.
Clams have been identified as an important alternate species for the future development of aquaculture in Atlantic Canada. One of the major obstacles in the development of clam culture has been controlling predators on culture sites, particularly endobenthic species (those that live in the sediment). In recent years, commercial size Quahaug and Soft-Shell Clam densities have reportedly been lower. While the cause for these declines has not yet been documented, harvesters have noted the important presence of predatory worms at clam harvesting sites. The Milky Ribbon Worm, Cerebratulus lacteus, is an important predator of many endobenthic bivalve species and its presence has been correlated to high field mortality in Soft-Shell Clams. Very little, however, is known about the factors regulating the patchy distribution of this predator. The present study will examine the factors regulating the patchy distribution and abundance of C. lacteus to allow for the development of predator management strategies.
Apr. 2014–Mar. 2017
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Innovation Fisheries Products Inc.; Mills Seafood Ltd.
Project Lead: Daniel Bourque (DFO)
Project Team: Angeline LeBlanc (DFO)
Collaborators: Innovation Fisheries Products Inc.; Mills Seafood Ltd.
Contact: Daniel.Bourque@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
QUAHOG (MERCENARIA MERCENARIA) SEEDSTOCK PRODUCTION FOR REMOTE SETTING IN NOVA SCOTIA
Quahog or hard clam (Mercenaria mercenaria) is an edible marine bivalve mollusc commonly found along the eastern shores of North America and Central America, ranging from Prince Edward Island to the Yucatán Peninsula. Due to strong market demand, there has been an interest to expand the production of quahogs in the Maritimes. The Gulf Aquaculture Association (GAA), based in Northumberland Strait, requested assistance from Nova Scotia Department of Fisheries and Aquaculture (NSDFA) and the Aquaculture Centre at Dalhousie University to assist the industry to improve quahog production in the province. Jesse Ronquillo (Principal Investigator) and Audrie-Jo McConkey (Shellfish Instructor and Algologist) devised innovative procedures in the spawning and larval rearing of quahog to produce the needed seedstock. Selected species of microalgae rich in highly unsaturated fatty acids (HUFA) were mass-produced continuously in an inventive way to provide abundant and diversified source of live feeds to larvae until they reach pediveliger or eyed-larvae that were used by shellfish growers in Northumberland Strait for remote setting. Over 97 million seedstock were produced in about a month for the grow-out operations of the shellfish growers in Nova Scotia.
The developed protocol for inducing broodstock spawning could improve quahog gamete production and simplify hatchery operations and management. The innovative technique for efficient and inexpensive culture of microalgae can be replicated as needed to improve commercial shellfish hatchery production and operations.
Mar. 2012–Oct. 2012
Funded By: Nova Scotia Department of Fisheries and Aquaculture (NSDFA) Co-Funded By: Gulf Aquaculture Association (GAA)
Project Lead: Jesse Ronquillo (UBC)
Project Team: Audrie-Jo McConkey (Dalhousie U)
Collaborators: Paul Budrewski (GAA); Ronakkumar Desai (Dalhousie U)
Contact: Jesse.Ronquillo@ubc.ca

Quahog spawning and seedstock production for remote setting. Photo: Jesse Ronquillo (UBC)
OCEAN ACIDIFICATION EFFECTS ON SHELLFISH AQUACULTURE
Rising atmospheric CO2 levels increases pCO2 in seawater, lowering the pH and causing ocean acidification which can profoundly affect shellfish aquaculture. Surface pCO2 can also rise in coastal regions due to the upwelling of deep, ancient water. While pCO2 levels are anticipated to rise consistently in the greater ocean due to elevated atmospheric CO2, coastal upwelling regions are already experiencing dramatic fluctuations in pCO2 that encompass the upper limits expected in the greater ocean in over 100 years’ time. Coincident with observations of enhanced pCO2 variation and potentially a higher average pCO2 experience along the BC and Washington State coastlines has been dramatic failures of shellfish aquaculture production both in the hatcheries and in ocean grow-out sites. This project was undertaken to begin collecting data and information in support of a causal link between reduced industry performance and pCO2, as well as to identify vulnerable developmental stages and physiologies.
A clear signature of development was observed in the microarray data for both scallops and oysters. However, while there appeared to be a negative growth response to elevated pCO2 during some time periods, there was no consistent gene expression pattern differentiating treatments in the microarray data. However, it was found that there may be some level of acclimation possible, i.e., individuals that survive adverse conditions as larvae will perform better under those conditions as juveniles. This may point to an alternate larval rearing strategy that may benefit industry through the rearing of larvae under sub-lethal adverse conditions and selecting the most robust for settling and ocean ranching.
Apr. 2011–Jul. 2013
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Island Scallops; Island Sea Farms Inc.; Taylor Shellfish Farms Canada; Limberis Seafoods Ltd.; Odyssey Shellfish Ltd.; Kyuquot Seafoods Ltd.
Project Lead: Kristi Miller-Saunders (DFO)
Collaborators: Island Scallops, Island Sea Farms Inc.; Taylor Shellfish Farms Canada; Limberis Seafoods Ltd.; Odyssey Shellfish Ltd.; Kyuquot Seafoods Ltd.
Contact: Kristi.Saunders@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm
COMPARING CULTURE GEAR AND ADAPTING OFF-SHORE GIANT SEA SCALLOP CULTURE HUSBANDRY TO BAIE DES CHALEUR, NEW BRUNSWICK
The availability of culture sites can be a limiting factor for the expansion of aquaculture in Canada. Up until 2014, most shellfish culture leases have been located in protected bays. However, in the Gulf Region, lease area in protected bays is limited. More importantly, the environmental factors (such as temperature) in protected bays are not suitable for shellfish like the Giant Sea Scallop (Placopecten magellanicus). This project assessed the growth and survival of Giant Sea Scallops cultured in an exposed environment (an offshore site in Baie des Chaleurs). Furthermore, the following five types of culture gear were tested in suspension to determine which gear best minimized scallop morality rates and promoted optimal growth rates: Pearl nets, Lantern nets, Dark Sea trays, OysterGro®, and Kenny cabins. In addition, scallops were also placed in OysterGro® on the bottom.
Results showed that scallops grown in suspension for two years had the highest growth rates except for those that were in heavily fouled gear. The gear with the best performance in terms of scallop growth rate and survival (0.078 ± 0.015 mm/day and 99.2%, respectively) was the Kenny cabin. Scallops grown in Lantern nets, which are the traditional culture gear, exhibited the highest growth rate (0.079 ±0.019 mm/day) but had the lowest survival (77.2%).
The results of this study have provided the information required to evaluate the potential to culture scallops offshore in Baie des Chaleurs and sustainably expand the shellfish aquaculture industry in this area. Based on the growth rates, survival, and spat collection rates observed in this study, farming sea scallop in an exposed environment is feasible.
Apr. 2010 –Mar. 2014
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Kenny Aquaculture
Project Lead: Leslie-Anne Davidson (DFO)
Collaborators: Kenny Aquaculture
Contact: Leslie-Anne.Davidson@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm

Scallop culture gear showing: a) Pearl net, b) Lantern net, c) Dark Sea trays, d) OysterGro® culture system with bottom floats, and e) Kenny cabin. Photo: Leslie-Anne Davidson (DFO)

Measuring scallops using electronic calipers that register data directly in an Excel file. Photo: Leslie-Anne Davidson (DFO)
DEVELOPING A CARRYING CAPACITY FRAMEWORK FOR BAYNES SOUND, BRITISH COLUMBIA
Shellfish aquaculture is an important activity in coastal environments. Successful culture of suspension-feeding shellfish relies heavily on the supply of phytoplankton food which is mainly controlled by hydrodynamic factors in estuaries with strong gradients in flushing characteristics (e.g., Baynes Sound, Strait of Georgia, B.C.). Large cultured bivalve populations may potentially deplete particles from the water column much faster than flushing or phytoplankton production can renew them on a local scale (farm depletion). Although re-suspension events or organic-rich sediments may provide a buffer against a fluctuating planktonic food supply, areas prone to re-suspension of silt-laden flocs may shutdown feeding of shellfish. The main objective of this project is to develop a carrying capacity model for shellfish production in Baynes Sound. The project will focus on establishing a particulate budget for the Sound and providing a real-time assessment of the current state of benthic and pelagic conditions in support of the development of siting criteria. Longer term goals involve developing circulation and nutrient models that describe the hydrodynamic and biological controls on particulate concentrations in Baynes Sound. These models will be coupled with shellfish assimilative estimates to determine the influence of shellfish production on benthic and water column quality.
Apr. 2011 – Mar. 2015
Funded By: DFO – Program for Aquaculture Regulatory Research (DFO – PARR)
Project Lead: Terri Sutherland (DFO)
Project Team: Peter Cranford, Chris Pearce, Hannah Stewart (DFO)
Contact: Terri.Sutherland@dfo-mpo.gc.ca
OPTIMIZING HATCHERY-BASED SEA SCALLOP SETTLEMENT
During the life cycle of bivalves, larvae transition from the pelagic (water-column) stages to benthic life via settlement and metamorphosis (physical development). Settlement is a significant limiting factor in the success of scallop hatcheries. Although settlement success in some larval cultures can reach up to 80% in good conditions, larval settlement and metamorphosis success rarely exceed 25 to 30%. Metamorphosis in bivalves is accompanied by the loss of their larval food collection system (velum) and the development of gills (adult food collection system). This period is critical given the larvae’s limited ability to feed while undergoing metamorphosis, and in order for metamorphosis to be successful, this change must be completed rapidly. Competent larvae will settle and undergo metamorphosis under the influence of various chemical, physical, and biological signals that are still unknown for Sea Scallops. This project aimed to increase settlement/metamorphosis success while reducing the time required to produce competent larvae.
Sea Scallops were successfully cultured in the production systems and this research has shown that it is possible to substantially increase settlement and metamorphosis success. By adjusting the hydrodynamic conditions of the production systems, it is possible to substantially increase settlement and metamorphosis success, the lack of which has been a major reason for losses in scallop hatcheries.
Apr. 2010–Oct. 2013
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Institut des Sciences de la Mer (ISMER); Société de Développement de l’Industrie Maricole (SODIM)
Project Lead: Jean-Marie Sévigny (DFO)
Project Team: Réjean Tremblay (Institute des Sciences de la Mer de Rimouski)
Collaborators: Institut des Sciences de la Mer (ISMER); Société de Développement de l’Industrie Maricole (SODIM)
Contact: Jean-Marie.Sevigny@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/science/enviro/aquaculture/acrdp-pcrda/index-eng.htm

Juvenile post-larval sea scallop, Placopecten magellanicus. Photo: Réjean Tremblay (UQAR)
DETERMINATION OF OPTIMAL MICROALGAL DIETS AND FEEDING RATIONS FOR LARVAE AND SEED OF THE GEODUCK CLAM (PANOPEA GENEROSA)
The geoduck clam aquaculture industry has been constrained by the lack of a reliable seed supply (due to very high larval mortality), indicating significant problems with the current hatchery production strategies. In order to establish hatchery-rearing protocols of a cultured bivalve species, it is necessary to examine a number of biotic and abiotic factors, such as temperature, diet quality, and diet quantity. This research aimed to determine optimal temperature, microalgal diets, and feeding rations for P. generosa larvae and seed.
Optimum microalgal diets were identified for larval and young juvenile geoduck clams. Optimum temperature and rations were identified for various size classes of young juvenile clams. These results will improve the reliability and production capacity of geoduck hatcheries in British Columbia and will help alleviate the hatchery bottleneck in the culture of geoduck clams. With improved hatchery knowledge, the aquaculture industry will be able to produce more geoduck seed (and more reliably) for outplanting.
Aug. 2010–Oct. 2013
Funded By: DFO – Aquaculture Collaborative Research and Development Program (DFO – ACRDP) Co-Funded By: Klahoose Shellfish Limited Partnership
Project Lead: Chris Pearce (DFO)
Project Team: Wenshan Liu, Ian Forster (DFO); Scott McKinley, Bianca Arney (UBC); Yichao Ren (Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao, China)
Collaborators: Klahoose Shellfish Limited Partnership
Contact: Chris.Pearce@dfo-mpo.gc.ca
www.dfo-mpo.gc.ca/aquaculture/acrdp-pcrda/index-eng.htm

Juvenile geoduck clams. Photo: Chris Pearce (DFO)
COMPARISON OF MUSCLE REFINING METHODS FOR SEA SCALLOPS (PLACOPECTEN MAGELLANICUS) FOR THE LAST TWO YEARS OF FARMING
Quebec scallop producers prefer suspension farming for growing Sea Scallop spat. This stage of production is conducted in pocket nets, lantern nets, or ear-hanging bags. Some previous studies have shown that using ear-hanging bags during the last two years yielded Sea Scallops with more muscle than pocket nets or lantern nets. However, scallops grown with ear-hanging bags are prone to significant biofouling that affect their survival and handling. The industry wants to have a production scenario that limits the biofouling while producing good quality muscle.
This project will provide biological data that Sea Scallop producers can use to optimize their production strategy. The project aims to evaluate Sea Scallop muscle refining scenarios. The specific objectives are to: 1) compare the yield in muscle of scallops grown using ear-hanging bags after two years with that of scallops grown using ear-hanging bags after one year; 2) document the effect of the density of scallops grown in lantern nets on muscle yield for two years; 3) document the effect of scallop position (lantern nets vs. ear-hanging bags) on muscle yield and activity; and 4) carry out a technical-economic analysis of selected production scenarios.
This project will generate useful data for scallop producers in order to optimize their production strategies.
Nov. 2012 – Dec. 2015
Funded By: MAPAQ
Project Lead: Carole Cyr (Merinov)
Project Team: Lisandre G. Solomon, Madeleine Nadeau (Merinov)
Contact: Carole.Cyr@merinov.ca

Filtration rate for scallops kept in a horizontal position (left) and in a vertical position (right). Photo: Carole Cyr (Merinov)

Sea cucumber (Parastichopus californicus) in tank at the DFO Pacific Biological Station. Photo: Emily Nelson
- Date modified: