Nocardiosis of Oysters
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
Nocardiosis, Fatal inflammatory bacteraemia (FIB), Focal necrosis, Multiple abscesses, Summer mortality, Pacific oyster nocardiosis (PON).
Scientific name or taxonomic affiliation
Nocardia crassostreae (Actinomycete bacteria) in the Nocardia otitidiscaviarum rRNA sub-group.
Geographic distribution
West coast of North America from the Strait of Georgia, British Columbia to California, and Japan (Matsushima Bay).
Host species
Crassostrea gigas and Ostrea edulis cultivated near infected C. gigas.
Impact on the host
Infection found all year but usually associated with mortalities during the late summer and fall. The extent of associated mortalities has not been accurately measured but estimated at about 35% in some localities. Friedman et al. (1999) found that oysters challenged with Nocardia synthesized heat shock proteins in a pattern similar to control animals. However, the degree of induced thermotolerance was reduced in oysters with nocardiosis.
Diagnostic techniques
Gross Observations
Round yellow-to-green pustules up to 1 cm in diameter on the surface of the mantle, gill, adductor muscle, and heart. The lesions can be reminiscent of those caused by Denman Island disease but are often larger in size.
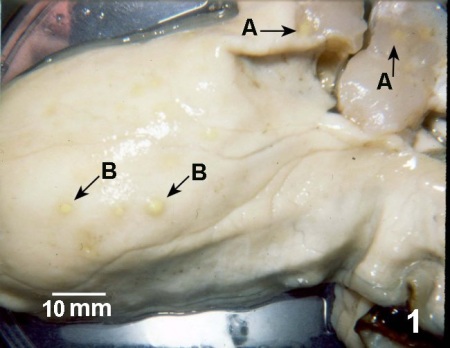
Figure 1. Crassostrea gigas with lesions in the adductor muscle (A) and body wall (B) caused by Nocardia sp.
Tissue Imprints
Gram-positive, acid-fast, branched and beaded colonies of filamentous bacteria are observed in imprints of material from pustules.
Histology
Dense clumps of deeply staining Gram-positive, PAS-positive, branching, beaded, basophilic bacteria surrounded by an intense accumulation of haemocytes in most organs.
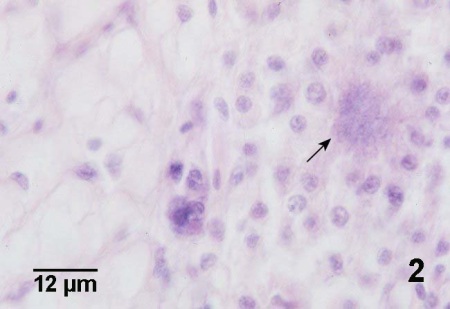
Figure 2. Histological section of Crassostrea gigas through the edge of a lesion in the mantle with normal vesicular connective tissue in the left part of the figure and an accumulation of haemocytes around a cluster of Nocardia sp. (arrow) on the right. This haematoxylin and eosin stained section does not reveal bacterial morphology.
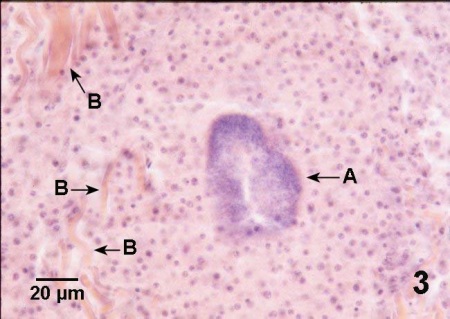
Figure 3. Histological section through a large lesion in the adductor muscle of Crassostrea gigas with nocardiosis. Massive accumulation of haemocytes around a colony of Nocardia sp. (A) has displaced and disrupted the muscle fibers (B) of the adductor muscle. Haematoxylin and eosin stain.
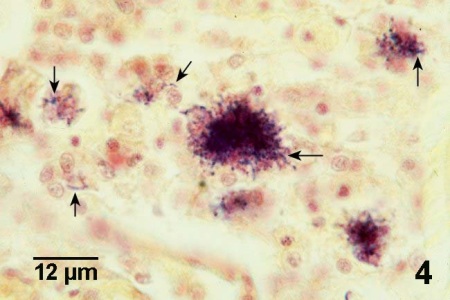
Figure 4. Gram stained histological section through lesion containing several clusters of Nocardia sp. (arrows) in Crassostrea gigas. Because Nocardia sp. are Gram-positive, the bacterium is more evident in Gram stained sections than in sections stained with haematoxylin and eosin stain (see Fig. 2 above for comparison).
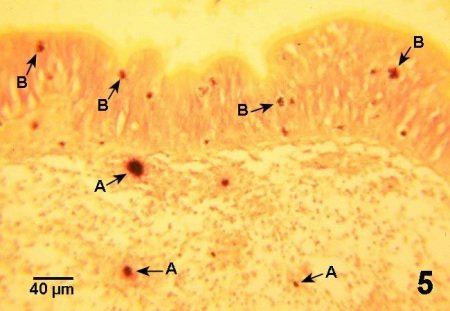
Figure 5. Histological section through wall of intestinal tract of Crassostrea gigas with nocardiosis. The clusters of Nocardia sp. are surrounded by haemocytes (A), many of which are in the process of migrating (B) through the epithelium of the gut wall. Gram stain.
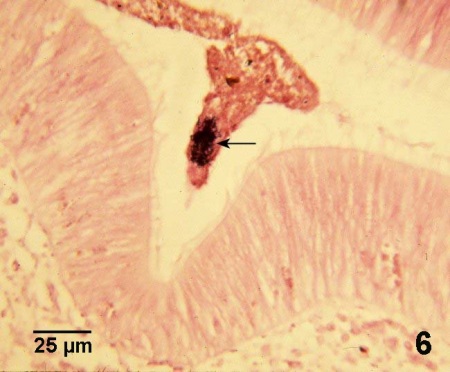
Figure 6. Cluster of Nocardia sp. (arrow) amongst the other gut contents in the lumen of the intestine of Crassostrea gigas, suggesting that oysters that are not too severely affected by nocardiosis can eliminate the bacteria and survive the infection. Gram stain.
DNA Probes
Two sets of PCR primers and one oligonucleotide probe for detecting PON were identified by Gee and Elston (1997). One primer set amplifies a portion of the 16S rDNA template from more than one genus of mycolic acid producing bacteria, including Nocardia. Nested in the first set is a primer set that is specific for the genus Nocardia. The oligonucleotide molecular probe is a pathogen specific sequence within the Nocardia specific amplicon. Primer sets and the probe can be used alone or in combination depending on the requirements for sensitivity and specificity. However, the trilaminar cell wall of N. crassostreae limits the amount of N. crassostreae DNA extracted from infected oysters when standard DNA extraction procedures are used (i.e., DNeasy Tissue Kit, Qiagen Inc. Mississauga, Ontario, Canada). Including a lysozyme treatment within the DNeasy extraction procedure significantly increased the yield of N. crassostreae DNA. Nevertheless, the PCR assay may be of similar sensitive for detecting nocardiosis in C. gigas as histological examination of tissue sections stained with Gram stain (Bower et al. 2005)
Culture
After 3-5 days of growth on Brain Heart Infusion (BHI) agar plates, strains isolated from diseased Pacific oysters produced a well developed mycelium that fragmented into irregular rod-shaped forms. The colonies were dry, waxy and wrinkled but did not carry aerial hyphae.
Methods of control
No known methods of treatment or control.
References
Bower, S.M., B. Goh, G.R. Meyer, R.B. Carnegie and A. Gee. 2005. Epizootiology and detection of nocardiosis in oysters. In: Walker, P.J., R.G. Lester, M.G. Bondad-Reantaso (eds.) Diseases in Asian Aquaculture V. Proceedings of the 5th Symposium on Diseases in Asian Aquaculture. Fish Health Section, Asian Fisheries Society, Manila. pp. 249-262.
Elston, R.A. 1993. Infectious diseases of the Pacific oyster Crassostrea gigas. Annual Review of Fish Diseases 3: 259-276.
Elston, R.A., J.H. Beattie, C. Friedman, R. Hedrick and M.L. Kent. 1987. Pathology and significance of fatal inflammatory bacteraemia in the Pacific oyster, Crassostrea gigas. Journal of Fish Diseases 10: 121-132.
Friedman, C.S. and R.P. Hedrick. 1991. Pacific oyster nocardiosis: isolation of the bacterium and induction of laboratory infections. Journal of Invertebrate Pathology 57: 109-120.
Friedman, C.S., H. Beattie, R. Elston and R.P. Hedrick. 1987. Isolation of the bacterium causing focal necrosis (fatal inflammatory bacteremia) in Pacific oysters (Crassostrea gigas). American Fisheries Society Fish Health Section Newsletter 15(1): 3.
Friedman, C.S., B.L. Beaman, R.P. Hedrick, J.H. Beattie and R.A. Elston. 1988. Nocardiosis of adult Pacific oysters, Crassostrea gigas. Journal of Shellfish Research 7: 216. (Abstract).
Friedman, C.S., J.H. Beattie, R.A. Elston and R.P. Hedrick. 1991. Investigation of the relationship between the presence of a Gram-positive bacterial infection and summer mortality of the Pacific oyster, Crassostrea gigas Thunberg. Aquaculture 94: 1-15.
Friedman, C.S., B.L. Beaman, J. Chun, M. Goodfellow, A. Gee and R.P. Hedrick. 1998. Nocardia crassostreae sp.nov., the causal agent of nocardiosis in Pacific oysters. International Journal of Systematic Bacteriology 48: 237-246.
Friedman, C.S., G.N. Cherr, J.S. Clegg, A.H. Hamdoun, J.L. Jacobsen, S.A. Jackson and K.R. Uhlinger. 1999. Investigation of the stress response, summer mortality and disease resistance of oysters, Crassostrea spp. Journal of Shellfish Research 18: 297. (Abstract).
Gee, A. and R.A. Elston. 1997. PCR detection of the bacterial pathogen in oyster nocardiosis. Journal of Shellfish Research 16: 264. (Abstract).
Imai, T., K. Mori, Y. Sugawara, H. Tamate, J. Oizumi and O. Itakawa. 1968. Studies on the mass mortality of oysters in Matsushima Bay VII. Pathogenic investigation. Tohoku Journal of Agricultural Research 25: 27-38.
Citation Information
Bower, S.M. (2006): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Nocardiosis of Oysters.
Date last revised: December 2006
Comments to Susan Bower
- Date modified: