Perkinsus marinus ("Dermo" Disease) of Oysters
On this page
Category
Category 1 (Not Reported in Canada)
Common, generally accepted names of the organism or disease agent
Perkinsus marinus, "Dermo" Disease, Proliferative disease, Perkinsosis.
Scientific name or taxonomic affiliation
Perkinsus marinus (=Dermocystidium marinum, =Labyrinthomyxa marina). The genus Perkinsus was originally placed in the Order Perkinsida of the Class Perkinsea within the Phylum Apicomplexa (Levine 1978). However, the conoid structure in the zoospores (as described below) is incomplete suggesting that the apicomplexan affinity is tenuous (Villalba et al. 2004). Taxonomic analysis based on nucleotide sequences indicate that Perkinsus may not belong in the Phylum Apicomplexa but seems to be more closely related to the Dinoflagellida (Goggin and Barker 1993, Perkins 1996, Siddall et al. 1997, Reece et al 1997b, Saldarriaga et al. 2003, Villalba et al. 2004). This hypothesis was supported by serological affinities of P. marinus with some dinoflagellates including a few free-living as well as parasitic species of Hematodinium from Nephrops norvegicus, Chionoecetes bairdi, Portunus pelagicus, Callinectes sapidus, Necora puber and an unidentified gammaridean amphipod (Bushek et al. 2002a). Norén et al. (1999) proposed that perkinsids, which share features with both dinoflagellates and apicomplexans, be described as a taxon on level with other alveolate phyla, with the phylum name of Perkinsozoa (Robledo et al. 2011, Mangot et al. 2011). Based on additional molecular analysis, Zhang et al. (2011a) supported the affiliation of the genus Perkinsus with an independent lineage (Perkinsozoa) positioned between the phyla of Apicomplexa and Dinoflagellata.
Geographic distribution
East coast of the United States of America (USA) from Maine to Florida (Andrews 1988,1996; Burreson and Ragone Calvo 1996; Ford and Tripp 1996), along the Gulf of Mexico coast to the Yucutan Peninsula (Burreson et al. 1994a, Aguirre-Macedo et al. 2007, Gullian-Klanian et al. 2008) and from the Paraíba River, northeastern Brazil (da Silva et al. 2013). The prevalence and intensity of infection varies with location and season (Craig et al. 1989, Crosby and Roberts 1990, Oliver et al. 1998b). "Hot spots" of infection have been reported within this range (Brousseau et al. 1998) and Perkinsus marinus poses a threat to oyster aquaculture in some areas (Ulrich et al. 2007). The Maryland, USA, Department of Natural Resources, Fisheries Service monitors the prevalence, intensity and distribution of P. marinus in Chesapeake Bay (Tarnowiski 2003, 2005). The range extension of this disease into Delaware Bay, New Jersey and Cape Cod, Maine, USA in about 1990, was attributed to repeated introductions by many means over many years in conjunction with an increases in sea-surface temperatures particularly during the winter (Ford 1996; Cook et al. 1998; Ford et al. 2000a, b; Karolus et al. 2000; Ford and Chintala 2006; Powell et al. 2008; Pecher et al. 2008). In the more northern locations, temperatures for parasite proliferation (higher than 20°C) are usually only suitable from June through September. Colder winters and high rainfall after 2002 reduced the prevalence of infection in some regions, but P. marinus can survive low temperatures and low salinities, and epizootic conditions are likely to return if temperatures rise again, as predicted by climate-change models (Ford and Smolowitz 2007). Perkinsus marinus was accidentally introduced into Pearl Harbor, Hawaii (Kern et al. 1973). In 2007, P. marinus was detected in cultured populations of endemic oysters (Crassostrea corteziensis) from the state of Nayarit on the Pacific coast of Mexico and these infections were associated with uncontrolled introductions of C. virginica in the region (Cáceres-Martínez et al. 2008, 2010). Subsequently, Cáceres-Martínez et al. (2012) detected P. marinus in natural and cultured populations of Saccostrea palmula from four coastal lagoons in the state of Sinaloa, on the Pacific coast of Mexico. Enríquez-Espinoza et al. (2010) attributed massive mortalities of farmed Crassostrea gigas in the Gulf of California (northwest Mexico) in July and August 2006 to P. marinus along with environmental factors. A Perkinsus-like parasite was reported in Crassostrea angulata imported into Great Britain from Portugal in the spring of 1969 (Alderman and Gras 1969). Perkinsus marinus as well as other species of Perkinsus were reported from Crassostrea rhizophorae and Crassostrea gasar (=brasiliana) in Brazil (Sabry et al. 2009, da Silva et al. 2012, da Silva et al. 2013, Brandão et al. 2013, Queiroga et al. 2013). One of the other species infecting C. rhizophorae from Ceará State, Northeastern Brazil was subsequently affiliated with Perkinsus beihaiensis based on phylogenetic analyses (Sabry et al. 2013).
Host species
Crassostrea virginica, Crassostrea corteziensis, Crassostrea rhizophorae, Saccostrea palmula and probably Crassostrea gasar (= brasiliana). Crassostrea gigas and Crassostrea ariakensis were also infected by experimental and environmental exposure but these two species seem to be more resistant to the disease (Barber 1996, Chu 1996, Calvo et al. 1999, Calvo et al. 2001, Paynter et al. 2008, Kingsley-Smith et al. 2009, Dungan et al. 2012). Schott et al. (2008) reported that during experimental field trials, the prevalence of P. marinus was equivalent to or higher in C. ariakensis than in native C. virginica and in the laboratory, C. ariakensis efficiently transmitted the parasite to uninfected C. virginica. Crassostrea rhizophorae was also experimentally infected and seemed to be as susceptible to infection as C. virginica but may be somewhat more tolerant to heavier parasite infections (Bushek et al. 2002c). da Silva et al. (2013) identified natural infections of P. marinus in over 70% of C. rhizophorae from the Paraíba River, northeastern Brazil. Cáceres-Martínez et al. (2008) reported a relatively low prevalence of natural infections (less than 6%) with no associated mortalities in two farmed populations of Crassostrea corteziensis from the Pacific coast of Mexico. Perkinsus marinus was also detected in another native oyster (Saccostrea palmula) from a state further south along the Pacific coast of Mexico but the light to moderate intensity of infection in up to 20% of the oysters may not reflect the susceptibility of this species of oyster to perkinsosis (Cáceres-Martínez et al. 2012). Clams, Mya arenaria and Macoma balthica were experimentally susceptible to infection by inoculations into the mantle cavity (Dungan et al. 2007). Coss et al. (2001) detected P. marinus DNA in the clam Macoma mitchelli. However, surveys, using sensitive and specific molecular assays, for P. marinus in clams (n=452 of five species) sympatric to (cohabitation with) C. virginica populations with high prevalences of P. marinus (50 to 100% infected) revealed P. marinus in only one clam (Mya arenaria) (Reece et al. 2008). Pecher et al. (2008) also reported that the prevalence of P. marinus in Mercenaria mercenaria was significantly lower than in C. virginica suggesting that M. mercenaria is not an optimal host for P. marinus. Aquacultured hard clams, M. mercenaria from the Gulf of Mexico coast of Florida, USA were negative for P. marinus by specific DNA probes (PCR) but low level infections were detected by Ray's thyoglycollate test and by genus-specific PCR (McCoy et al. 2007). Perkinsus marinus was also reported from the ectoparasitic snail Boonea impressa which was reported to transmit P. marinus between C. virginica in the laboratory (White et al. 1987). Note that C. virginica can also be infected by other species of Perkinsus (Coss et al. 2001, Pecher et al. 2008). In addition, two other species of Perkinsus (P. mediterraneus and P. beihaiensis) have been described from other species of oysters from European and Asian waters, respectively. Also, similar Perkinsus spp. have been reported in the rock oyster Saccostrea forskali cultured in Thailand (Taveekijakarn et al. 2005) as well as in at least 34 species of molluscs (including clams, abalone and scallops) from warm temperate to tropical waters of the Atlantic and western Pacific oceans and Mediterranean Sea, in 30 species of bivalves on the Great Barrier Reef, and in cultured Pinctada maxima (pearl oysters) from Torres Strait, Australia (Norton et al. 1993, Perkins 1996). Mussels such as Mytilus edulis and Geukensia demissa, not known to develop perkinsosis, contained high anti-P. marinus activity in their haemolymph (Anderson and Beaven 2001).
Impact on the host
The endoparasite Perkinsus marinus has caused mass mortalities of C. virginica and is one of the primary factors that adversely impacts the abundance and productivity of this oyster at various locations along the east and Gulf coasts of the USA (Burreson and Ragone Calvo 1996, Ford 1996, Ray 1996, Powell et al. 2008). For example, the large decrease in oyster production in the Maryland part of Chesapeake Bay between 1981 and 1988, with landings as low as 15,000 metric tons since 1986 (compared to landings fluctuating around 80,000 metric tons between about 1910 and 1980) was attributed to high mortalities related to infections with P. marinus and Haplosporidium nelsoni, predation and poor management practices (Héral et al. 1990, Goulletque et al. 1994). However, the significant declines in C. virginica production in this area prior to 1950 was probably attributed to the mechanical destruction of habitat and stock over-fishing and not disease (Rothschild et al. 1994). Carnegie and Burreson (2008) noted that in the Virginia part of Chesapeake Bay, C. virginica populations persisted despite being infected with P. marinus and have a capacity for growth if substrate is managed effectively and the loss of reefs arrested. In Delaware Bay, C. virginica populations that are highly susceptible to P. marinus survive in the upper bay refugia possibly because environmental conditions inhibit infection (Pydeski and Bushek 2008). Gullian-Klanian et al. (2008) indicated that in Mexico, no serious epizootics attributed to P. marinus have been reported. Although high prevalences (mean of 70%) occurred in Terminos Lagoon (Mexico, Gulf of Mexico) during the dry season, the intensity of infection was usually light. Klanian et al. (2008) suggested that freshwater input associated with high nutrient concentrations during the rainy and north-wind seasons had a strong negative effect on P. marinus prevalence (23% and 7%, respectively) and also influenced the physiology of the oysters and that this seasonal stress was responsible for the absence of an epizootic event in Terminos Lagoon.
The life cycle of P. marinus includes trophozoites in the water column entering the paleal cavity of the oyster during filter-feeding and subsequently being directed through gills and palps towards the mouth. Marine aggregates (small clumps of material suspended in the water column) that contained P. marinus produced higher infection intensities than freely suspended P. marinus (Ralph et al. 2008) and important tissue sites of infection were the pallial organ (labial palps) and the principal pseudofaeces discharge area of the mantle (Winnicki et al. 2008). In agreement, Allam et al. (2013) reported that infection commonly occurs in the pseudofeces discharge area of the mantle during the rejection of material before reaching the mouth and P. marinus cells in aggregates caused significantly higher disease prevalence and infection intensities when compared to freely-suspended parasite cells. Espinosa et al. (2013) suggested that the mucus from the mantle of C. virginica plays a significant role in the pathogenesis of P. marinus by enhancing the proliferation and the infectivity of this parasite. Once in the paleal cavity or the digestive tract, trophozoites displaying surface ligands for the oyster galectin Crassostrea virginica galectin (CvGal) (Tasumi and Vasta 2007) are recognised and phagocytosed by the haemocytes that can transmigrate to the internal milieu and eventually into the vascular system. Parasites reside inside phagosome-like vesicles of the haemocytes where they remain viable and multiply (tomonts). When infected haemocytes disintegrate, the released trophozoites can either be phagocytosed by neighbouring haemocytes or multiply extracellularly as tomonts in both the internal milieu and lumen of the vascular system. The infected circulating haemocytes migrate throughout the host tissues where they lyse and release trophozoites, leading to systemic infection and eventually host death. Trophozoites are released into the water from live oysters via the feces and/or the pseudofeces and upon death of the oyster, from the decaying infected tissues (Bushek et al. 2002b). Once released into the water column, trophozoites may sporulate: trophozoites enlarge (prezoosporangia), develop a discharge tube and after multiple rounds of division, release hundreds of zoospores into the water column. Whether zoospores develop into trophozoites remains an open question (Robledo et al. 2011).
The effects of P. marinus infection in C. virginica range from pale appearance of the digestive gland, reductions in condition index, impaired gametogenic development, reduced haemolymph protein concentrations and lysozyme activity, to severe emaciation, gaping, shrinkage of the mantle away from the outer edge of the shell, retarded growth and occasionally the presence of pus-like pockets (Ford and Tripp 1996). The impact of P. marinus at varying infection intensities on the energy budget of the oyster can be estimated and explained with the acquisition of energy by P. marinus production and respiration (Choi et al. 1989). Ford et al. (1999) reported that P. marinus distributions within host populations were aggregated (for example, 1 or 2 oysters may contain more parasites than all other oysters in the sample). Remacha-Triviño et al. (2008) determined that the mean total number of trophozoites in eight naturally infected C. virginica was about 11.5 million cells with about 97% as mature trophozoites (signet rings), 2% as immature trophozoites (meronts) and 1% as clusters of small immature trophozoites (tomonts or merozoites). The percentage of trophozoites detected in various tissues was: intestine (30.1%), vesicular connective tissue (21.3%), haemocytes (14.9%), digestive gland (11.4%), gills (6.1%), connective tissues (5.7%), gonads (4.1%), palps (2.2%), muscle (1.9%), mantle connective tissue (0.8%), pericardium (0.7%), mantle epithelium (0.1%), and heart (0.1%) (Remacha-Triviño et al. 2008).
Proliferation of the parasite causes systemic disruption of connective tissue and epithelial cells and is correlated with warm summer water temperatures (higher than 20°C) when pathogenicity and associated mortalities are highest. In South Carolina, the prevalence and intensity P. marinus in C. virginica was highest during periods of oyster spawning which also corresponded with warmer temperatures (Burrell et al. 1984, Bobo et al. 1989). However, in this area, relationships between salinity, temperature and gonadal stage of development of the oyster to infection with P. marinus were not evident (Burrell et al. 1984). Nevertheless, differences in salinity were found to effect the prevalence and intensity of P. marinus in C. virginica in Chesapeake Bay (Paynter et al. 1993a). Also, in the mid northern region of the Gulf of Mexico, the intensity and prevalence of infection was correlated with salinity (Mackin 1955, Soniat et al. 2012) but not with water temperature (Soniat and Gauthier 1989, Gauthier et al. 1990). Infected C. virginica can eliminate viable P. marinus with the faeces and pseudofaeces at a rate correlated to both P. marinus body burden and subsequent survival time and may be important in transmission before infections become lethal. However, in an epizootic, shedding of P. marinus via faeces and pseudofaeces is relatively small compared to the potential number released by cadavers of heavily infected oysters (Bushek et al. 2002b). Hoese (1964) reported that snails, crabs and fish that scavenge on dead oyster tissues may serve as vectors of P. marinus after passive transfer of the parasite through the gut of the scavenger.
Saunders et al. (1993) determined that the growth rate of P. marinus in C. virginica was dependent on P. marinus population density and that this parasite could control its own population level but the delicate balance could be destabilized resulting in epizootics. Some C. virginica may survive summer proliferation of the parasite but are unable to revive following over-wintering dormancy. Mortalities of up to 95% have occurred in C. virginica during the second summer following transfer to disease enzootic areas. Although some researchers have reported inhibition of gonadal development and decline in reproductive output in infected oysters, Dittman et al. (2001) found that in Delaware Bay, the very drastic negative effect on reproduction predicted during the gametogenic phase did not materialize at the time of spawning because a period of diminishing parasite burdens coincided with gamete maturation. This observation was confirmed for C. virginica in Chesapeake Bay by Carnegie and Burreson (2011a). However in laboratory experiments, Chintala et al. (2002) found increased levels of infection in oysters undergoing spawning and/or exposed to low oxygen stress. Willson and Burnett (2000) did not find a strong correlation between oxygen uptake and infection intensity with P. marinus at either 25 or 35°C. However, Breitburg et al. (2011) reported that diel-cycling hypoxia increased acquisition of P. marinus infections, most likely by reducing the oyster defence responses but decreased the release of infective stages possibly because of lower filtration rates and consequently lower faeces production.
Perkinsus marinus incorporates and modifies lipids and synthesises phospholipids from exogenous sources and the metabolic modes of trophozoites (meronts) differ from those of prezoosporangia (Chu et al. 1998, 1999; Lund and Chu 2002). Unlike other protistan parasites, trophozoites of P. marinus are capable of synthesizing long chain essential fatty acids de novo (specifically, unsaturated fatty arachidonic acid 20:4(n-6) from acetate) in vitro via a delta-8 pathway (Chu et al. 2002, 2004). Venegas-Calerón et al. (2007) examined the genetics associated with this process. Perkinsus marinus is an agent of stress on its host as assessed by the biochemical measurement of the taurine-to-glycine ratio in C. virginica (Soniat and Koenig 1982). Infection decreased taurine and glycine levels by about 40% and decreased free amino acid levels by about 33% thereby possibly impairing the salinity tolerance mechanisms of C. virginica (Paynter et al. 1993b, 1995). Although haemocytes from infected C. virginica had a greater zymosan-induced chemiluminescence (Anderson et al. 1992, 1993, 1995), P. marinus trophozoites did not stimulate a chemiluminescence response in oyster haemocytes (Anderson 1999a). This suggests that P. marinus may be able to suppress or remove the release of reactive oxygen intermediates from haemocytes, thus evading this component of the host's defence despite the capability of the oyster haemocytes to recognize and phagocytize P. marinus (Volety and Chu 1995, La Peyre et al. 1995b, Anderson 1999b, Schott et al. 2003a). One mechanism that may be employed by P. marinus to abrogate the respiratory burst by haemocytes (the host's oxidative defence response) following phagocytosis is the dismutation of superoxide radicals to molecular oxygen and hydrogen peroxide by superoxide dismutases (SODs) described by Asojo et al. (2006) and Fernández-Robledo et al. (2008a) in P. marinus as iron-cofactored PmSOD1 and PmSOD2. In vitro challenges showed that haemocytes isolated from C. virginica can kill about 50% of P. marinus from cultured isolates (Volety and Fisher 2000). However, P. marinus produces many extracellular proteins (ECP) in vitro and there is evidence that these ECP, especially proteases, are important in vivo for the establishment of infection, defence parameters of the oyster and propagation of the parasite (Garreis et al. 1996; La Peyre and Faisal 1997a; Volety and Chu 1997; La Peyre and Cooper 1998; Oliver et al. 1998a, 1999b, b; La Peyre and Volety 1999; Ottinger et al. 2001; Wright et al. 2002; Ahmed et al. 2003; Muñoz et al. 2003; Schott and Vasta 2003; Schott et al. 2003b; Jordan and Deaton 2005). Also, EPC activity is affected by temperature (Chu et al. 2003) and suppresses the vibriocidal activity of oyster haemocytes to effectively eliminate the bacterium Vibrio vulnificus, potentially leading to conditions favouring higher numbers of vibrios in oyster tissues (Tall et al. 1999). Robledo et al. (2004) identified the complementary DNA (cDNA) sequence of the divalent cation transporter natural resistance–associated macrophage protein homologue from P. marinus (PmNramp) that may relate to the parasite's survival of intracellular killing by the host.
Antimicrobial activity against P. marinus occurs in cell-free haemolymph (plasma) of oysters (Anderson and Beaven 2000). Although serum agglutinins were not associated with resistance to P. marinus (Chintala et al. 1994), low molecular weight protein inhibitors (such as the slow-tight binding serine protease inhibitor described by Xue et al. (2006)) and high antiproteolytic activity in the haemolymph of C. virginica and C. gigas may play a role in oyster host defence mechanisms (Oliver et al. 1999a, 2000; Faisal 1999; Elsayed and Faisal 1999; Xue et al. 2006; La Peyre et al. 2010a). Yu et al. (2011) and He et al. (2012) indicated that polymorphism in the serine protease inhibitor gene (at cvSI-1) was associated with resistance to P. marinus in C. virginica. Nitric oxide production by oyster haemocytes was also found to have a role in decreasing parasite loads at early time points after infection (Villamil et al. 2007). Chu and La Peyre (1989) found no linkage between haemolymph lysozyme and protein concentration and infection of oysters by P. marinus. However, C. virginica at low temperature and low salinity had higher plasma lysozyme concentrations which may result in an unfavourable environment for the development of the parasite and/or weaken parasite activity (Chu and Volety 1997a, Chu 1998). La Peyre et al. (2010b) indicated that in vitro, the largest decreases in P. marinus viability occurred only with combined low temperature and low salinity, indicating that there is clearly a synergistic effect between these environmental parameters on the parasite. The role (if any) of heat shock proteins in stress and disease resistance in oysters requires further investigation (Brown et al. 1993, Tirard et al. 1995, Encomio and Chu 2005). However, the heat shock response in C. virginica was not negatively affected by P. marinus infection (Encomio and Chu 2007). Wang et al. (2010) employed microarray analysis of gene expression in C. virginica to reveal a novel combination of antimicrobial and oxidative stress host responses 30 days after challenge with P. marinus.
Bushek and Allen (1996a) found that races of C. virginica resistant to P. marinus exist and that natural levels of resistance roughly corresponded to the extent ancestral populations had been exposed to P. marinus. Also, differential selection by P. marinus can generate divergence in popluation structure in C. virginica (Green-Beach et al. 2011). Sokolova et al (2006) found two arbitrary fragment length polymorphism (AFLP) markers in C. virginica from North Corolina, USA, that were associated with infection and suggested the existence of genes or groups of genes that act directly or indirectly to control the levels of infection. Bushek and Allen (1996b) also indicated that races of P. marinus vary in virulence and environmental tolerance. The occurrence of different P. marinus strains with distinct phenotypic traits was confirmed by Yee et al. (2005) and microsatellite analysis of P. marinus genotypes from Florida and New Jersey indicated limited parasite migration between geographically proximate populations of oysters (Thompson et al. 2008). Reece et al. (2001a, b) detected 12 different composite genotypes of P. marinus with >88% of 76 isolates possessing one of three predominant genotypes but they found that a single oyster can be infected with multiple strains.
Modeling of diseased C. virginica populations by Hofmann et al. (1995) and Powell et al. (1996) showed that food availability to the host and salinity and temperature control on the growth and development rates of the host and parasite are environmental factors involved in regulating P. marinus. Temperature, salinity and their interaction are important environmental influences on transmission and pathogenicity of P. marinus in C. virginica (Crosby and Roberts 1990, Audemard et al. 2006). However, other environmental and biological factors (e.g., pollution, other human influences, host nutrition and growth, spawning and reproduction, age, resistance, oyster density and distribution and disease vectors) affect the levels of parasitism observed in the field (Craig et al. 1989, Soniat 1996). For example, Ragone Calvo et al. (2003b) suggested that atypical early-summer oyster mortality from Haplosporidium nelsoni, at a time when infections of P. marinus are light, can have a significant indirect influence on P. marinus transmission dynamics. Also, a relationship with changes in salinity caused by climatic cycles was associated with changes in disease prevalence and intensity (Soniat et al. 2006, 2009, 2012). Lenihan et al. (1999) and Volety et al. (2000) demonstrated that the depth below mean low water at which the oyster occurs on the reef affects the prevalence and intensity of infection and oyster mortality with the most significant impact on oysters towards the base of the reef. Also, land use patterns (anthropogenic disturbances) may affect the distribution of the disease and exacerbation of oyster mortalities (White et al. 1998, Bushek et al. 2000b, Power et al. 2006). For example, in the USA, Gulf of Mexico, Wilson et al. (1990, 1992) reported that latitude, total petrolium aromatic hydrocarbon (PAH) content and industrial and agricultural land use significantly affected the parasite's distribution and concluded that industrial and agricultural activity may correspond with high prevalence and intensity of disease caused by P. marinus.
Exposure of the oyster to pollutants such as the chemical carcinogen n-nitrosodiethylamine (DENA) and tributyltin (TBT) will enhance the disease caused by P. marinus (Winstead and Couch 1988 and Anderson et al. 1996, respectively). Also, exposure of C. virginica to field contaminated sediments and related water soluble fractions for about one month in the laboratory significantly elevated the expression/ progression of latent P. marinus infections in a dose-dependent manner (Chu and Hale 1994, Chu 1999). However, Burreson and Ragone Calvo (1996) claim that there is little evidence to support the common perception that pollution is responsible for the dramatic increase in P. marinus abundance since 1985. An assessment of the impact of several common anthropogenic contaminants on the proliferation of P. marinus in vitro determined that only a herbicide (with active ingredients of 3.1% 2,4-dichlorophenoxyacetic acid, 10.6% mecoprop and 1.3% dicamba that mimic growth hormones of broadleaf plants to over stimulate growth resulting in plant death) had a significant negative effect, but only above the manufacture's recommended application rate (Bushek et al. 2007). Foster et al. (2011) reported that copper (as CuCl2) reduced growth of P. marinus in vitro and greatly reduced infection levels of haemocytes in vivo, presumably by direct toxic effects on the parasite.
Crassostrea gigas and triploid C. ariakensis were found to be less susceptible (but not completely resistant) to infection and disease caused by P. marinus (Meyers et al. 1991; Calvo et al. 1999, 2000, 2001 ). However, C. gigas may be less tolerant to environmental factors prevailing in usual C. virginica habitat (Barber and Mann 1994, Chu 1996). Greater resistance to infection and disease in C. gigas may be attributed to elevated cellular and humoral activities (including protease inhibitors) that may degrade the parasite more effectively, and/or lower plasma protein levels that may limit parasite growth (La Peyre et al. 1995a, Romestand et al. 2002, Goedken et al. 2005). Espinosa et al. (2013) reported that growth of P. marinus was dramatically inhibited in in vitro cultures that were supplemented with pallial mucus of C. gigas but was significantly enhanced by mantle mucus from C. virginica in comaprison to cultures enriched with the other supplemental extracts. Also, differences in gene expression in challenged C. virginica and C. gigas were identified by suppression subtractive hybridization (Tanguy et al. 2004). Although sterile triploid C. virginica grew faster and had a lower cumulative mortality than diploids, the triploids were equally susceptible to P. marinus (Barber and Mann 1991, Dégremont et al. 2012). Advanced infections observed in C. ariakensis exposed to P. marinus in the laboratory suggests that there may be some risk of mortality to C. ariakensis if this oyster is held under stressful conditions at least in hatchery or laboratory settings (Moss et al. 2006)
Diagnostic techniques
Gross Observations
Infected oysters may have a pale appearance to the digestive gland, reductions in condition index, severe emaciation, gaping, shrinkage of the mantle away from the outer edge of the shell, reduced gonadal development and/or retardation of growth. Occasionally, pus-like pockets may occur in the soft tissues. However, these signs are not pathognomonic of perkinsosis. Cáceres-Martínez et al. (2008) reported that infection in C. corteziensis was associated with gross signs of weakness and transparent and retracted mantle.
Wet mount
Spherical bodies containing an eccentric vacuole ("signet-ring") in preparations from moribund oysters. Because other species of Perkinsus share this morphological feature, other methods will have to be employed to determine the specific identity of organisms. Cheng and Manzi (1996) indicated that P. marinus could be detected in haemolymph of infected oysters following the application of a "panning" technique (specifically, about 1 milliliter of haemolymph was placed on the bottom of a Petri dish, incubated at 25°C for 30 minutes followed by examination of the nonadhering cells for parasites).
Histology
Because the infection is usually systemic, the connective tissue of all organs may harbour immature trophozoites (= meronts, merozoites or aplanospores, 2-3 µm in diameter), mature trophozoites (= "signet-ring" stages or mature meronts, merozoites, trophonts or aplanospores, 3-10 µm in diameter each containing a large eccentric vacuole (possibly containing a vacuoplast) that presses the nucleus to the periphery of the cell) and tomonts (="rosettes", sporangia or schizonts, 4 -15 µm in diameter and containing 2, 4, 8, 16 or 32 developing immature trophozoites). Many of the parasites may occur within oyster haemocytes. Trophozoite morphology does not have taxonomic value because it can be influenced by the host, the time of the year, and nutrient availability (Villalba et al. 2004). For example, Carnegie and Burreson (2011b) reported that since 1986, large trophozoites of P. marinus at relatively low infection intensities in C. virginica from Chesapeake Bay have been replaced by abundant minute parasite cells. Histopathology of severe infections in C. corteziensis, as well as other susceptible oysters, consists of general invasive infiltration of haemocytes, including phagocytosis of parasite stages, that were disseminated in the connective tissue surrounding the epithelia of the digestive gland, gonad and mantle with the presence of some brown cells (Cáceres-Martínez et al. 2008).
Figures 1 to 3. Perkinsus marinus in histological sections of the connective tissue in the digestive gland (Figs. 1 to 3) and intestinal epithelium (Fig. 4) of Crassostrea virginica from Maryland, USA. Haematoxylin and eosin stain.
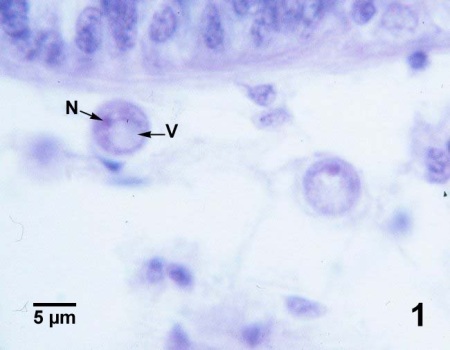
Figure 1. Two mature trophozoites ("signet ring" stage). The large eccentric vacuole (V) and nucleus (N) are indicated on the specimen closest to the wall of a digestive gland tubule.
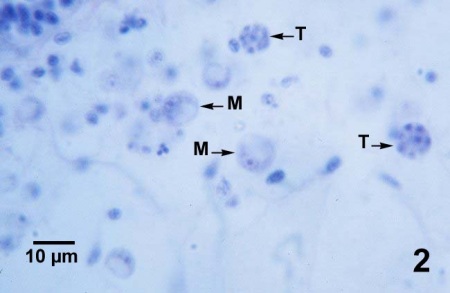
Figure 2. Mature trophozoites (M) in which the basophilic chromatin of the nucleus appears as a ring around the perimeter of the nucleus, and two eight-cell tomonts (T) within which immature trophozoites are developing.
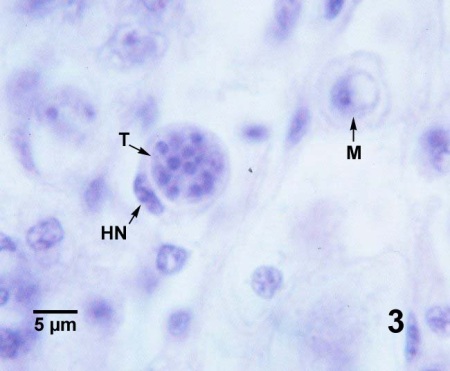
Figure 3. A 16-cell tomont (T) containing developing trophozoites. This tomont is contained within a haemocyte (HN indicates the nucleus of the phagocytic cell) and a maturing trophozoite (M) is located near by.

Figure 4. Heavy infection consisting of trophozoites (Z), tomonts (T) and mature trophozoites (M).
Electron Microscopy
A marked characteristic of all species of Perkinsus is the ultrastructure of the zoospore. For P. marinus, zoospores have been produced by transferring the prezoosporangium from thioglycollate medium (see cultures below) into sea water where it develops into a zoosporangium that produces hundreds of motile biflagellate zoospores. The anterior flagellum is ornamented with hair-like structures (mastigonemes) and spurs-like structures and the posterior flagellum is glabrous. The zoospore contains an apical complex consisting of a conoid, subpellicular microtubules, rhoptries, rectilinear micronemes and conoid-associated micronemes. Large vacuoles also occur at the anterior end of the zoospore. (For ultrastructural details and illustrations of zoospores see Perkins 1976b and Perkins 1996). Perkins (1969) described the ultrastructure of vegetative stages as found in the host C. virginica. Sunila et al. (2001) described the ultrastructural characteristics of P. marinus vegetative stages (trophozoites (=trophonts) and tomonts (=schizonts)) from in vitro propagation cultures, and zoosporangia isolated from C. virginica haemolymph, enlarged in Fluid Thioglycollate Medium (as described below) and zoosporulated in Dulbecco's Modified Eagle/Ham's F-12 liquid medium supplemented with 3% fetal bovine serum and antibiotics. Fernández-Robledo et al. (2008a) depicted that the P. marinus superoxide dismutase 2 (PmSOD2) was localizes to single-membrane, vesicle-like subcellular compartments that fuse and/or discharge their content into a larger double-membrane compartment.
Immunological Assay
Polyclonal antibodies that recognized only prezoosporangia (=hypnospores) of P. marinus (Choi et al. 1991) and others that bind to most life stages of many but not all species of Perkinsus as well as other parasitic dinoflagellates and monoclonal antibodies that recognize epitopes unique to the prezoosporangia have been produced (Dungan and Roberson 1993, Bushek et al. 2002a). Other monoclonal antibodies produced by Romestand et al. (2001) detected P. marinus trophozoites and their protein lysates and also trophozoites from Perkinsus olseni (=atlanticus) from European clams (Ruditapes decussatus). Immunoassays have been successfully applied to various environmental samples and in vitro experimental systems (Dungan 1997). Polyclonal antibodies produced to purified P. marinus extracellular proteins recovered from defined culture medium were used to produce an ELISA-based assay that may be comparable to Ray's thioglycollate test as described below (Kaattari et al. 1997, Dungan and Hamilton 1997, Ottinger et al. 2001). Remacha-Triviño et al. (2008) used stereological methods (defined as a collection of strongly based mathematical procedures aimed to quantify geometrical properties of target objects without assumptions concerning inherent characteristics of these objects) and immunohistochemistry (using the rabbit anti- P. marinus IgG of Dungan and Roberson (1993)) to quantify and determine the tissue distribution of P. marinus in C. virginica.
DNA Probes
Small subunit ribosomal RNA gene (SSU rDNA, consisting of at least 1,793 nucleotides) has been sequenced by polymerase chain reaction (PCR) and molecular cloning. Specific and sensitive semiquantitative, quantitative and competitive PCR assays, including multiplex PCR (simultaneous testing of two or more pathogens in a single test reaction), and quantitative real-time PCR assays were developed for: 1) assessing intensity (including quantification) of P. marinus in oysters, 2) determining prevalence of infections in oyster populations, 3) monitoring of over-wintering oyster populations, 4) certifying disease free oyster spat, 5) elucidating mechanisms of infection, 6) assessing the presence of the parasite in environmental waters and various invertebrate species and 7) differentiating between various species of Perkinsus (Marsh et al. 1995; Vasta et al. 1997; Penna et al. 1999, 2001; Yarnall et al. 2000; Burreson 2000a; Russell et al. 2000, 2004; Elandalloussi et al. 2004; Abollo et al. 2006; Audemard et al. 2004, 2006; Pecher et al. 2008). Robledo et al. (1998), Yarnall et al. (2000) and Gauthier et al. (2006) described various PCR based assays to detect P. marinus that proved to be more sensitive than Ray's thioglycollate test (described below). Inter- and intra-specific genetic variation among Perkinsus species has provided the opportunity to design genus- and species-specific molecular diagnostic assays (Reece et al. 2001a, b). However, before molecular analysis (e.g., PCR) can be recommended as the method of choice for disease diagnosis, more research is necessary to validate the various molecular diagnostic assays and compare them to standard diagnostic techniques (Burreson 2000a, b; Reece and Burreson 2004; Villalba et al. 2004). Apparently, the real-time quantitative PCR assay developed and validated by De Faveri et al. (2009) correlated with traditional detection methods (Ray's thioglycollate test) and did not amplify Perkinsus chesapeaki or Perkinsus olseni DNA. A loop-mediated isothermal amplification (LAMP) assay was designed to target the conserved internal transcribed spacer 2 (ITS2) region of the SSU rRNA gene of Perkinsus spp. (Feng et al. 2013). Although this LAMP assay was apparently validated using clam samples collected from coastal areas in eastern China known to be infected with Perkinsus olseni, Feng et al. (2013) also claimed that it detected P. marinus in oysters imported from Australia where the parasite is not known to occur according to the World Health Organisation for Animal Health Manual of Diagnostic Tests for Aquatic Animals 2013 and Australia's National List of Reportable Diseases of Aquatic Animals 2011.
Molecular sequence data is playing an increasingly important role in the identification of Perkinsus species and requires adequate DNA sequence data at the targeted loci from the same and related species over a wide geographic area in order to develop reliable, accurate and sensitive molecular diagnostic tools (Villalba et al 2004). Thirteen percent difference in the internal transcribed spacer 1 (ITS1) plus internal transcribed spacer 2 (ITS2) of the SSU rDNA between P. marinus and 4 other isolates of Perkinsus (P. olseni (=atlanticus) from clams in Portugal, P. olseni from abalone in Australia, isolates of Perkinsus sp. from the jewel box and blood cockle in Australia) is a strong indication that P. marinus of C. virginica is a distinct species. Nevertheless, Brown et al. (2004) found 14 polymorphic nucleotide positions at the ITS region (8 in ITS1 and 6 in ITS2) in 12 isolates of P. marinus from the Atlantic and Gulf of Mexico coasts of the USA and indicated the importance of determining the genetic variation of each locus prior to development of sequence-based molecular diagnostics. The use of PCR primers to amplify up to six polymorphic loci of genomic DNA from cultured P. marinus indicated that in vitro P. marinus are diploid and that oysters may be infected by multiple strains of this parasite (Reece et al. 1997a, c, 1999). The serine protease gene in P. marinus is variable and may correlate with its virulence or pathogenicity (Brown and Reece 2000, 2001, 2003). Allele sequences were identified in isolates from geographically distant sites. However, allelic and genotypic frequencies differed significantly among isolates from regions of the northeast and southeast US Atlantic coast and the coast of the Gulf of Mexico (Reece et al. 1999). Primers that target the non-transcribed spacer (NTS), a region with high inter-specific variation, have demonstrated good species specificity (Robledo et al. 1998), although intra-specific variations were detected and the prevalence of the two described types varied with the geographic origin of the samples (Robledo et al. 1999). However, intra-specific variations within the NTS region has not been broadly assessed creating a risk of false negatives due to polymorphism within a species if the PCR primers do not bind the target sequence of all strains of that species (Villalba et al. 2004). Assessing high resolution microsatellite markers and amplified alleles directly from infected oyster genomic DNA, Thompson et al. (2011) suggested that P. marinus employs multiple reproductive modes, and that over the short term, selection acts upon independent parasite lineages rather than upon individual loci in a cohesive, interbreeding population. Nevertheless, high genotypic diversity is the evolutionary legacy of sex in P. marinus. Anthropogenic movement of infected oysters may increase outcrossing opportunities, potentially facilitating rapid evolution of this parasite (Thompson et al. 2011). Other components of the P. marinus genome have been described but to date, none of these have been developed into diagnostic assays (Stelter et al. 2007; Fernández-Robledo et al. 2008b; Matsuzaki et al. 2008; Joseph et al. 2010; Zhang et al. 2011 a, b; Hearne and Pitula 2011; Robledo et al. 2011).
Culture
Examine tissues for blue-black prezoosporangia (= hypnospores, usually 30-80 µm in diameter but extremes of 480 µm in diameter have been observed), after incubation in Fluid Thioglycollate Medium (FTM) supplemented as described by Ray (1966) for approximately 7 days followed by staining with Lugol's iodine stain, (see Ray (1966), Choi et al. (1989), Fisher and Oliver (1996) and Kim et al. (2006) for details of the technique and see Nickens et al. (2002) for an alternative formulation). This diagnostic procedure is frequently referred to as Ray's thioglycollate test (or technique, Ray's FTM, RFTM). Although not true propagating cultures, this procedure is used for the diagnosis of many species of Perkinsus but may also detect other organisms (Villalba et al. 2004). The oyster tissue that is assayed for parasite surveys or monitoring work tend to reflect the same seasonal trend but the choice of tissue is important depending on the accuracy required if oyster body burden of the parasite is being assessed (Bushek et al. 1994, Oliver et al. 1998b). Methods to improve determination of the intensity of infection, (whole-oyster parasite burden) described by Fisher and Oliver (1996), have been suggested by Coates et al. (1999). Ray's thioglycollate test has been used to quantify the intensity of infection without sacrificing the oyster by determining the number of parasites in a haemolymph sample (Gauthier and Fisher 1990; Nickens et al. 2000b, 2002). The use of Ray's thioglycollate test to detect and quantify planktonic P. marinus in environmental water samples has been proposed by Ellin and Bushek (1999, 2000b, 2006). However, other methods will have to be employed to determine the specific identity of organisms that may stain with Lugol's iodine following incubation in FTM. Audemard et al. (2008) indicated that P. marinus DNA could be successfully amplified from samples processed by Ray's thioglycollate test including staining with Lugol's iodine stain. Thus, the identity of the Perkinsus sp. in a positive Ray's thioglycollate test can be determined with the added advantage of being able to preserve positive samples in 95% ethanol for later processing for DNA amplification and sequencing or submission to a laboratory with molecular identification capabilities (Audemard et al. 2008).
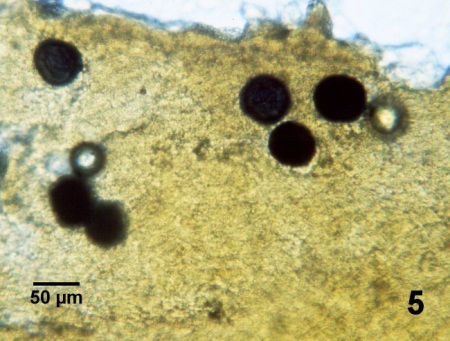
Figure 5. Wet mount of the rectum of Crassostrea virginica that was processed by the Ray's thioglycollate test (including staining with Lugol's iodine) to reveal six enlarged and darkly (blue-black) staining prezoosporangium of Perkinsus marinus.
Methods for the in vitro propagation of histozoic stages of P. marinus were described by Kleinschuster and Swink (1993), La Peyre et al. (1993), Gauthier and Vasta (1993, 1995), La Peyre and Faisal (1995a, 1995b, 1996), La Peyre (1996), and Soudant and Chu (2001). La Peyre and Chu (1994) described a simple procedure for the isolation of P. marinus trophozoites (merozoites) from heavily infected C. virginica. The in vitro culture of this parasite has led to insights into its biology. Krantz (1994) used in vitro cultures to assay chemicals for inhibitory activity and to screen for potential chemotherapeutic agents. Perkinsus marinus cultured in nutrient media were sensitive to low salinity with mortalities increasing as salinity decreased below 12 ppt (Burreson et al. 1994b). By culturing P. marinus in media of various osmolarities (168 to 737 mOsm - equivalent to 6.5 to 27.0 ppt), O'Farrell et al. (2000) determined that the size of P. marinus vegetative stages varied with osmolarity and cells cultured at low osmolarity can withstand hypoosmotic (56 mOsm - 2.5 ppt) and hyperosmotic (672 mOsm - 24.7 ppt) stress (41% mortality at 2.5 ppt) where as cells cultured at high osmolarity experienced 100% mortality when transferred to the hypoosmotic artificial seawater. La Peyre et al. (2006) reported that P. marinus exhibited reduced viability at 7 ppt, even after acclimation. Lund et al. (2004) found that in culture media, salinity treatments (about 14, 20 and 28 ppt) exhibited few treatment effects, but temperature significantly affected cell proliferation, fatty acid content and fatty acid synthesis rates (i.e., fatty acid synthesis rates increased approximately two-fold for every 10°C increase in temperature). Ford and Chintala (2006) used in vitro data to test the hypothesis that the northward expansion of P. marinus was associated with a low-temperature adapted strain of the parasite. They found no evidence of low-temperature adaptation by P. marinus based on the fact that net proliferation rates for isolates were similar at temperatures from 5 to 20°C. However, at temperatures of 25 to 35°C, the South Carolina isolates exhibited higher proliferation rates than the northern isolates suggesting possible high-temperature adaptation of parasite strains that are routinely exposed to higher temperatures. La Peyre et al. (2008b) determined that P. marinus had 49% viability after 30 days at 4°C, but limited metabolic activity and no proliferation which could partially explain decreasing parasite infection intensities in C. virginica during the colder months of the year. Using in vitro assays, Soudant and Chu (2001) determined that during immature trophozoite proliferation, P. marinus synthesizes certain fatty acids and lipid classes, but for development from immature trophozoites to prezoosporangium, the parasite may rely on its host for lipid resources. For example, Lund et al. (2007) suggested that P. marinus cannot synthesize sterols and must sequester them from its host. Also, extracts or haemolymph from C. ariakensis, C. gigas and some non-oyster mollusc species significantly reduced in vitro proliferation compared to extracts from C. virginica (Gauthier and Vasta 2002, Brown et al. 2005). Tissue extracts from C. virginica caused P. marinus to secrete elevated amounts of a set of low molecular weight serine proteases (LMP: 30–45 kDa) which were not upregulated by extracts from C. gigas and C. ariakensis (MacIntyre et al. 2003).
Five percent fetal bovine serum was required in one culture media formulation but higher concentrations dramatically reduce parasite proliferation in a dose-dependant manner because of transferrin (a natural iron chelator) within the serum which sequesters available iron resulting in P. marinus growth inhibition (Gauthier and Vasta 1994). An in vitro tetrazolium-based cell proliferation assay was described for monitoring the affect of conditions on P. marinus multiplication (Dungan and Hamilton 1995). Shridhar et al. (2013) adapted a commercial adenosine tri-phosphate (ATP) content-based assay to assess the in vitro proliferation of P. marinus in a 96-well plate format, and validated the method by measuring the effects of potential anti-proliferative compounds. La Peyre and Faisal (1997b) described a protein-free chemically defined culture medium and proposed that the medium was suitable for the study of P. marinus proteins, to produce antigens for antibody production and to screen chemotherapeutic agents. Bushek et al. (2000a) described a technique to clone P. marinus using micromanipulation and a "feeder layer".
Perkinsus marinus produced in vitro were infective to oysters by injection into the shell cavity or adductor muscle but not via feeding. Ford et al. (2002) reported that virulence was lost immediately in culture and repassing cultured P. marinus through oysters did not restore virulence. Also, P. marinus inlog-phase were significantly more virulent than those from lag- or stationary-phase cultures (Ford et al. 2002, Chintala et al. 2002). Although, cultured P. marinus appeared to have a low pathogenicity (Bushek et al. 1997a, 2002b), supplementing the cultures with oyster plasma or tissue homogenates seemed to enhance infectivity (Earnhart et al. 2004) and led to pronounced changes in P. marinus cellular morphology comparable to those observed within naturally infected oysters (MacIntyre et al. 2003). Shaheen (1999) determined the P. marinus can survive in artificial seawater at 22 ppt salinity and 27 °C for more that 6 weeks without an exogenous supply of nutrients. Cryopreserved isolates of P. marinus are available at the American Type Culture Collection (ATTC, Rockville, MD, USA).
Methods of control
Oysters from areas with records of the disease should not be imported into Canada. Perkinsus marinus is easily transmitted between oysters, thus, it is imperative to avoid moving infected oysters to an area containing uninfected oysters (Ford and Tripp 1996). To date, eradication has proven impossible. Management methods used to reduce the commercial impact of the disease on infected populations consist of reducing the density of oysters and harvesting or moving oysters to low salinity areas (lower than 9 ppt (Ragone and Burreson 1993)) before water temperatures increase to 15-20°C (however, infections may persist for years in low salinity areas (Burreson and Ragone Calvo 1996)). From the results of in vitro experiments on P. marinus cultured at different salinities, O'Farrell et al. (2000) suggested that transferring infected oysters to low salinity will result in strains of P. marinus acclimated to low salinity and thus able to withstand periodic events of extremely low salinity. Thus, caution is necessary when using low salinity areas to treat or control infection and/or disease (Paynter and Burreson 1991). However, repetitive and well-timed low salinity (freshet) events can prevent infection or at least maintain P. marinus at non-lethal intensities making the control of freshwater inflows a possible adaptive management approach (Mackin 1955, La Peyre et al. 2003, 2009). Specifically, low salinity events (less than 5 ppt) decreased P. marinus infection intensities, even as temperatures exceeded 20°C (La Peyre et al. 2009). This relationship between P. marinus and salinity was used by authorities in coastal areas of Texas as a biological indicator for determining minimum freshwater inflows (Culbertson et al. 2011). Nevertheless, Fisher et al. (1992) and Chu and Volety (1997b) found that temperatures (between 10°C and 28°C) was more influential than salinity (3 to 39 ppt) in affecting susceptibility of oysters to P. marinus, the intensity of P. marinus infections and oyster mortalities. The relationship between changes in salinity associated with climate change and changes in disease prevalence and intensity suggested an approach for predicting epizootics of P. marinus from climate models and thus be used in the management of oyster populations (Soniat et al. 2006).
Chu and Greene (1989) used prezoosporangia and zoospores obtained from Ray's thyoglycollate test to determine that prezoosporangia cannot withstand temperatures as low as 4°C for more than 4 days and zoospores died in 1 day when transferred from 28°C to 4°C. Cold winter temperatures may limit the natural spread of this pathogen to northern areas (infection declines at temperatures below 15-20°C). However, oysters held for 11 weeks at 15°C, a temperature considered more favourable for oyster haemocytes than for P. marinus, were not able to eliminate infections (Ford et al. 1999). In enzootic areas, strategies designed to enhance and supplement natural recruitment of oysters, along with keeping growing areas free from P. marinus by limiting oyster transplantation, currently offer the most promise for maintaining commercially harvestable stocks (Krantz and Jordan 1996). Paynter et al. (2010) determined that hatchery-produced juvenile oysters planted on numerous natural oyster bar in Maryland between 1995 and 2009 grew well enough to reach market size in 2 to 3 years and the rates of P. marinus infection were low. Thus, they suggested that disease-related mortality would not often threaten oyster aquaculture within the area (Paynter et al. 2010). Management strategies of fallowing beds after removing infected oysters has not proven effective in some areas but early harvest to avoid mortalities caused by P. marinus may be feasible (Butsic et al. 2000). La Peyre et al. (2008a) suggested that oysters cultured off-bottom on adjustable long-line systems and exposed to air daily had increased survival and higher condition indices. Ford et al. (2000c, 2001) demonstrated that juvenile oysters (seed) from nursery systems that use raw water pumped from an enzootic area are highly likely to be infected although infections may be very light in intensity and low in prevalence. Treating raw water, by filtration to 1 µm and then exposure to ultraviolet light (30,000 µW s-1 cm-2 UV irradiation), will help protect hatchery produced seed from infection (Ford et al. 2001). Deploying specific-pathogen-free C. virginica into enzootic areas may not reduce infection and subsequent mortalities, and ultimate success with production will depend on the salinity regime they experience during grow-out (Albright et al. 2007).
Efforts to identify Crassostrea virginica stocks resistant to Perkinsus marinus are in progress (Bushek and Allen 1996a, b; Stickler et al. 2001; Encomio et al. 2005, Wang and Guo 2008). Some disease-tolerant strains of C. virginica had better growth and survival than other strains (Abbe et al. 2010) and wild oysters from disease prone locations could contribute to the development of disease resistance in farmed oysters (Roberts et al. 2008). Although, stocks selected for resistance to other pathogens (i.e., Haplosporidian nelsoni) were highly susceptible to P. marinus (Burreson 1991), dual resistance to both parasites was achieved through four generations of artificial selection at a location where both diseases are enzootic (lower York River, Virginia, USA) (Ragone Calvo et al. 2003a). Also, interline crossing of C. virginica stocks developed for resistance to P. marinus, H. nelsoni and Roseovarius sp. seemed to perform well under a variety of disease pressures (Rawson et al. 2008). Yu and Guo (2006) detected post-mortality shifts in genotype frequency linked to Dermo/summer mortality-resistance quantitative trait loci (QTLs) and suggested that QTLs were candidate genome regions for further analysis to understand genetic mechanisms of disease-resistance and for the development of genetically improve cultured stocks. Powell et al. (2011) used a gene-based population dynamics model to assess the apparent limited development of resistance by C. virginica to P. marinus and determined that a mortality rate that limits the development of disease resistance still strains the ability of the species to maintain a vibrant population necessary to its long-term survival. Specifically, a limited infusion of susceptible larvae might be sufficient to offset any selective advantage realized from an epizootic mortality rate of 20 to 25%.
Based on the results of in vitro and in vivo studies, Faisal et al. (1999) suggested that bacitracin (a family of branched cyclopeptide antibiotics produced by Bacillus licheniformis and B. subtilis and used to treat various bacterial and protozoan infections in vertebrates) has promise for use in P. marinus chemotherapy. Lund et al. (2005) and Chu et al. (2008) suggested that the antimicrobial drug triclosan (5-chloro-2-(2,4 dichlorophenoxy) phenol, a specific inhibitor of Fab1 (enoyl-acyl-carrier-protein reductase) an enzyme in the Type II class of fatty acid synthetases) may be effective in treating P. marinus-infected oysters. However, quinine (a traditional preventative and treatment for the malarial parasites Plasmodium spp.) was lethal to infected oysters at concentration below the effective concentrations for P. marinus trophozoies (meronts) in vitro or had no observable effect on parasite infections (Panko et al. 2008). Shridhar et al (2013) adapted a commercial bioluminescent adenosine tri-phosphate (ATP) content-based assay and used it to assess the in vitro effects of various potential anti-proliferative drugs against P. marinus. Foster et al. (2011) suggested that the ability of copper (as CuCl2) to greatly reduce infection levels of P. marinus in haemocytes in vivo may be useful as a potential therapeutic against Dermo disease in aquaculture conditions.
Although standard bleach added to filtered seawater with diluted chlorine concentrations of 300 parts per million (ppm) killed P. marinus after 30 minutes, chlorine tolerance of P. marinus in culture media was significantly greater, and this parasite in tissues survived 2100 ppm chlorine making chlorine treatment of P. marinus contaminated materials prior to ocean disposal ineffective (Bushek et al. 1997b, c). The quarantine of oyster shells on land for at least one month can dramatically reduce the potential risk of spreading P. marinus when using oysters shells (e.g., as cultch) from contaminated areas (Bushek et al. 2004). Apparently, one hour exposure to fresh water or one hour incudation of cultured parasites in sea water or culture media at 50°C killed P. marinus but about one hour at 60°C was required to kill the parasites within tissues (Bushek et al. 1997b, c). Bushek and Howell (2000) reported that UV sterilizer units could be used as a practical and economical means to treat effluents and prevent or at least minimize the transmission of P. marinus to local oyster populations from infected oysters that are in facilities such as processing plants, depuration facilities, hatcheries, laboratories, etc. Low to moderate doses of UV irradiation (4,000 to 14,000 μWs/cm2) inhibited the proliferation of P. marinus and higher doses (greater than 28,000 μWs/cm2) lead to parasite mortality with the higher the dosage, the more effective the sterilization (Bushek and Howell 2000). Organic N-halamine disinfectants (up to 25 mg/L for up to 12 h exposure, depending on the specific chemical formulation) can also be used to disinfect seawater contaminated with P. marinus (Delaney et al. 2003).
Seasonal proliferation of P. marinus has been modeled to estimate time to critical levels and duration of infection in C. virginica (Hofmann et al. 1995, 1999; Soniat and Kortright 1998; Brewster et al. 1999, 2000; Ragone Calvo and Burreson 2000; Brousseau and Baglivo 2000a, b; Ragone Calvo et al. 2001). One model has been developed into an internet program that may assist in calculating the time to a critical level of disease (Soniat et al. 2000, Ray et al. 2001; see advertisement by Scarratt 2000). Simulations of the model developed by Hofmann et al. (1999) could be used to understand the causes underlying the northward spread of the disease and to restructure the practices of the oyster industry to maximize production under conditions where the life span of the commercial species is controlled by disease. The model of Ragone Calvo et al. (2001) suggests that a single transmission event may be sufficient for P. marinus to become enzootic in a specific year class of oysters located in moderate to high salinity areas, while periodic transmission events are required for the parasite to persist in low salinity areas. Field studies by McCollough et al. (2007) confirmed the development of epizootics within 8 weeks of exposure to local infection pressures, when first infections were simultaneously detected among greater than 62% of the specific-pathogen-free juvenile C. virginica experimentally deployed into a mesohaline enzootic area. A model for managing the oyster fishery during times when disease is a controlling influence was developed and assessed using oyster populations affected by P. marinus (Klink et al. 2001). Jordan (1995) used cluster (multivariate classification) analysis to evaluate oyster population structure and disease dynamics of some Maryland C. virginica populations.
References
Abbe, G.R., C.B. McCollough, L.S. Barker and C.F. Dungan. 2010. Performance of disease-tolerant strains of eastern oyster (Crassostrea virginica) in the Patuxent River, Maryland, 2003 to 2007. Journal of Shellfish Research 29: 161-175.
Abollo, E., S.M. Casas, G. Ceschia and A. Villalba. 2006. Differential diagnosis of Perkinsus species by polymerase chain reaction-restriction fragment length polymorphism assay. Molecular and Cellular Probes 20: 323-329.
Aguirre-Macedo, M.L., R.A. Simá-Álvarez, M.K. Román-Magaña and J.I. Güemez-Ricalde. 2007. Parasite survey of the Eastern oyster Crassostrea virginica in coastal lagoons of the southern Gulf of Mexico. Journal of Aquatic Animal Health 19: 270–279.
Ahmed, H., E.J. Schott, D.J. Gauthier and G.R. Vasta. 2003. Superoxide dismutases from the oyster parasite Perkinsus marinus: purification, biochemical characterization, and development of a plate microassay for activity. Analytical Biochemistry 318: 132-141.
Albright, B.W. and G.R. Abbe. 1999. Recent trends in infection of the eastern oyster Crassostrea virginica by the parasite Perkinsus marinus in the Patuxent River estuary. Journal of Shellfish Research 18: 317. (Abstract).
Albright, B.W., G.R. Abbe, C.B. McCollough, L.S. Barker and C.F. Dungan. 2007. Growth and mortality of dermo-disease-free juvenile oysters (Crassostrea virginica) at three salinity regimes in an enzootic area of Chesapeake Bay. Journal of Shellfish Research 26: 451-463.
Alderman, D.J. and P. Gras. 1969. "Gill Disease" of Portuguese oysters. Nature 224: 616-617.
Allam, B., W.E. Carden, J.E. Ward, G. Ralph, S. Winnicki and E.P. Espinosa. 2013. Early host-pathogen interactions in marine bivalves: Evidence that the alveolate parasite Perkinsus marinus infects through the oyster mantle during rejection of pseudofeces. Journal of Invertebrate Pathology 113: 26-34.
Anderson, R.S. 1996. Interactions of Perkinsus marinus with humoral factors and hemocytes of Crassostrea virginica. Journal of Shellfish Research 15: 127-134.
Anderson, R.S. 1999a. Lack of hemocyte chemiluminescence stimulation by Perkinsus marinus in eastern oysters Crassostrea virginica with dermo disease. Journal of Aquatic Animal Health 11: 179–182.
Anderson, R.S. 1999b. Perkinsus marinus secretory products modulate superoxide anion production by oyster (Crassostrea virginica) haemocytes. Fish and Shellfish Immunology 9: 51-60.
Anderson, R.S. and A.E. Beaven. 2000. Antimicrobial activity in cell-free hemolymph of oysters and mussels. Journal of Shellfish Research 19: 641. (Abstract).
Anderson, R.S. and A.E. Beaven. 2001. A comparative study of anti-Perkinsus marinus activity in bivalve sera. Journal of Shellfish Research 20: 1011-1017.
Anderson, R.S., K.T. Paynter and E.M. Burreson. 1992. Increased reactive oxygen intermediate production by hemocytes withdrawn from Crassostrea virginica infected with Perkinsus marinus. The Biological Bulletin (Woods Hole, Mass.) 183: 476-481.
Anderson, R.S., L.L. Brubacher, L.M. Mora, K.T. Paynter and E.M. Burreson. 1993. Hemocyte responses in Crassostrea virginica infected with Perkinsus marinus. Journal of Shellfish Research 12: 135. (Abstract).
Anderson, R.S., E.M. Burreson and K.T. Paynter. 1995. Defense responses of hemocytes withdrawn from Crassostrea virginica infected with Perkinsus marinus. Journal of Invertebrate Pathology 66: 82-89.
Anderson, R.S., M.A. Unger and E.M. Burreson. 1996. Enhancement of Perkinsus marinus disease progression in TBT-exposed oysters (Crassostrea virginica). Marine Environmental Research 42: 177-180.
Andrews, J.D. 1988. Epizootiology of the disease caused by the oyster pathogen Perkinsus marinus and its effects on the oyster industry. American Fisheries Society Special Publication 18: 47-63.
Andrews, J.D. 1996. History of Perkinsus marinus, a pathogen of oysters in Chesapeake Bay 1950-1984. Journal of Shellfish Research 15: 13-16.
Andrews, J.D. and S.M. Ray. 1988. Management strategies to control the disease caused by Perkinsus marinus. American Fisheries Society Special Publication 18: 257-264.
Asojo, O.A., E.J. Schott, G.R. Vasta and A.M. Silva. 2006. Structures of PmSOD1 and PmSOD2, two superoxide dismutases from the protozoan parasite Perkinsus marinus. Structural Biology and Crystallization Communications, Section F, Acta Crystallographica 62: 1072-1075.
Audemard, C., K.S. Reece and E.M. Burreson. 2004. Real-time PCR for detection and quantification of the protistan parasite Perkinsus marinus in environmental waters. Applied and Environmental Microbiology 70: 6611-6618.
Audemard, C., L.M. Ragone Calvo, K.T. Paynter, K.S. Reece and E.M. Burreson. 2006. Real-time PCR investigation of parasite ecology: in situ determination of oyster parasite Perkinsus marinus transmission dynamics in lower Chesapeake Bay. Parasitology 132: 827-842.
Audemard, C., R.B. Carnegie and E.M. Burreson. 2008. Shellfish tissue evaluation for Perkinsus spp. using the Ray's fluid thioglycolate medium culture assay can be used for downstream molecular assays. Diseases of Aquatic Organisms 80: 235-239.
Barber, B.J. 1996. Gametogenesis of eastern oysters, Crassostrea virginica (Gmelin, 1791), and Pacific oysters, Crassostrea gigas (Thunberg, 1793) in disease-endemic lower Chesapeake Bay. Journal of Shellfish Research 15: 285-290.
Barber, B.J. and R. Mann. 1991. Sterile triploid Crassostrea virginica (Gmelin, 1791) grow faster than diploids but are equally susceptible to Perkinsus marinus. Journal of Shellfish Research 10: 445-450.
Barber, B.J. and R. Mann. 1994. Growth and mortality of eastern oysters, Crassostrea virginica (Gmelin, 1791), and Pacific oysters, Crassostrea gigas (Thunberg, 1793) under challenge from the parasite, Perkinsus marinus. Journal of Shellfish Research 13: 109-114.
Bobo, M.Y., J.J. Manzi and V.G. Burrell. 1988. Perkinsus marinus: temporal and environmental aspects of infection in South Carolina oyster populations. Journal of Shellfish Research 7: 571. (Abstract).
Bower, S., E. Burreson and K. Reece. 2003. Annex 10: Review of molecular techniques used to differentiate the various species/isolates of Perkinsus. Report of the Working Group on Pathology and Diseases of Marine Organisms, Aberdeen, UK, 11-15 March 2003. Mariculture Committee, ICES CM 2003/F:03, Ref. ACME, pg. 54-60.
Brandão, R.P., G. Boehs, R.C. Sabry, L.O. Ceuta, M.d.S.A. Luz, F.R. Queiroga and P.M. da Silva. 2013. Perkinsus sp. infecting oyster Crassostrea rhizophorae (Guilding, 1828) on the coast of Bahia, Brazil. Journal of Invertebrate Pathology 112: 138-141.
Brewster, J., D. Bushek and R.F. Dame. 1999. An ecosystem model of Perkinsus marinus. Journal of Shellfish Research 18: 326-327. (Abstract).
Brewster, J., D. Bushek and R. Dame. 2000. Perkinsus marinus population dynamics in North Inlet, South Carolina - an ecosystem model. Journal of Shellfish Research 19: 660. (Abstract).
Breitburg, D., D. Hondorp, C. Audemard, R. Carnegie, R. Burrell and V. Clark. 2011. Breathless nights: diel-cycling hypoxia and the prevalence of Perkinsus marinus (Dermo) infections in Chesapeake Bay oysters. Journal of Shellfish Research 30: 488. (Abstract).
Brousseau, D.J. 1996. Epizootiology of the parasite, Perkinsus marinus (Dermo) in interdial oyster populations from Long Island Sound. Journal of Shellfish Research 15: 583-587.
Brousseau, D.J. and J.A. Baglivo. 2000a. Modeling seasonal proliferation of the parasite, Perkinsus marinus (Dermo) in field populations of the oyster, Crassostrea virginica. Journal of Shellfish Research 19: 133-138.
Brousseau, D.J. and J.A. Baglivo. 2000b. Perkinsus disease progression in field oysters: a modeling study. Journal of Shellfish Research 19: 660. (Abstract).
Brousseau, D.J., J.C. Guedes, C.A. Lakatos, G.R. Lecleir and R.L. Pinsonneault. 1998. A comprehensive survey of Long Island Sound oysters for the presence of the parasite, Perkinsus marinus. Journal of Shellfish Research 17: 255-258.
Brown, G.D. and K.S. Reese. 2000. Identification of a serine protease gene in Perkinsus marinus. Journal of Shellfish Research 19: 660. (Abstract).
Brown, G.D. and K.S. Reece. 2001. Variations in serine protease gene(s) among Perkinsus marinus isolates. Journal of Shellfish Research 20: 538. (Abstract).
Brown, G.D. and K.S. Reece. 2003. Isolation and characterization of serine protease gene(s) from Perkinsus marinus. Diseases of Aquatic Organisms 57: 117-126.
Brown, D.C., B.P. Bradley and K.T. Paynter. 1993. The physiological effects of protozoan parasitism on the eastern oyster, Crassostrea virginica: induction of stress proteins. Journal of Shellfish Research 12: 135-136. (Abstract).
Brown, G.D., S. Kotob and M. Faisal. 1999. Diversity among Perkinsus marinus isolates from the Chesapeake Bay. Journal of Shellfish Research 18: 317. (Abstract).
Brown, G.D., K.L. Hudson and K.S. Reece. 2004. Multiple polymorphic sites at the ITS and ATAN loci in cultured isolates of Perkinsus marinus. Journal of Eukaryotic Microbiology 51: 312–320.
Brown, G.D., S.L. Kaattari and K.S. Reece. 2005. Effect of homogenate from different oyster species on Perkinsus marinus proliferation and subtilisin gene transcription. Journal of Shellfish Research 24: 1027-1033.
Burrell, V.G., M.Y. Bobo and J.J. Manzi. 1984. A comparison of seasonal incidence and intensity of Perkinsus marinus between subtidal and intertidal oyster populations in South Carolina. Journal of the World Mariculture Society 15: 301-309.
Burreson, E.M. 1991. Effects of Perkinsus marinus infection in the eastern oyster, Crassostrea virginica: I. Susceptibility of native and MSX-resistant stocks. Journal of Shellfish Research 10: 417-423.
Burreson, E.M. 2000a. Molecular diagnostics for the oyster pathogens Haplosporidium nelsoni (MSX disease) and Perkinsus marinus (Dermo disease) in Chesapeake Bay, Virginia, USA. In: Walker, P. and R. Subasinghe (eds.) DNA-based Molecular Diagnostic Techniques. Research Needs for Standardization and Validation of the Detection of Aquatic Animal Pathogens and Diseases. FAO Fisheries Technical Paper. No. 395: 71-78, (for complete FAO publication of Technical Paper No. 395 for on-line version of paper by Burreson see http://www.fao.org/docrep/005/X4946E/x4946e0i.htm#bm18).
Burreson, E.M. 2000b. Disease diagnosis by PCR: foolproof or fool hardy? Journal of Shellfish Research 19: 642. (Abstract).
Burreson, E.M. and L.M. Ragone Calvo. 1996. Epizootiology of Perkinsus marinus disease of oysters in Chesapeake Bay, with emphasis on data since 1985. Journal of Shellfish Research 15: 17-34.
Burreson, E.M., R.S. Alvarez, V.V. Martinez and L.A. Macedo. 1994a. Perkinsus marinus (Apicomplexa) as a potential source of oyster Crassostrea virginica mortality in coastal lagoons of Tabasco, Mexico. Diseases of Aquatic Organisms 20: 77-82.
Burreson, E.M., L.M. Ragone Calvo, J.F. La Peyre, F. Counts and K.T. Paynter. 1994b. Acute osmotic tolerance of cultured cells of the oyster pathogen Perkinsus marinus (Apicomplexa: Perkinsida). Comparative Biochemistry and Physiology 109A: 575-582.
Bushek, D. and S.K. Allen. 1996a. Host-parasite interactions among broadly distributed populations of the eastern oyster Crassostrea virginica and the protozoan Perkinsus marinus. Marine Ecology Progress Series 139: 127-141.
Bushek, D. and S.K. Allen. 1996b. Races of Perkinsus marinus. Journal of Shellfish Research 15: 103-107.
Bushek, D. and T.L. Howell. 2000. The effect of UV irradiation on Perkinsus marinus and its potential use to reduce transmission via shellfish effluents. Northeastern Regional Aquaculture Center (NRAC) Publication No. 00-008. North Dartmouth, Massachusetts, USA, 5p.
Bushek, D., S.E. Ford and S.K. Allen. 1994. Evaluation of methods using Ray's fluid thioglycollate medium for diagnosis of Perkinsus marinus infection in the eastern oyster, Crassostrea virginica. Annual Review of Fish Diseases 4: 201-217.
Bushek, D., S.K. Allen, K.A. Alcox, R.G. Gustafson and S.E. Ford. 1997a. Response of Crassostrea virginica to in vitro cultured Perkinsus marinus: preliminary comparisons of three inoculation methods. Journal of Shellfish Research 16: 479-485.
Bushek, D., R. Holley and M. Kelly. 1997b. Chlorine tolerance of Perkinsus marinus. Journal of Shellfish Research 16: 260. (Abstract).
Bushek, D., R. Holley and M. Kelly. 1997c. Treatment of Perkinsus marinus-contaminated materials. Journal of Shellfish Research 16: 330. (Abstract).
Bushek, D., A.J. Erskine, R.F. Dame, L.D. Coen and N. Hadley. 1999. Transmission of Perkinsus marinus to intertidal oysters. Journal of Shellfish Research 18: 317-318. (Abstract).
Bushek, D., R.A. Holley and K.S. Reece. 2000a. Use of micromanipulation and "feeder layers" to clone the oyster parthogen Perkinsus marinus. Journal of Eukaryotic Microbiology 47: 164-166.
Bushek, D., J. Keesee, B. Jones, D. White, J. Neet and D. Porter. 2000b. Shellfish health management: a system level perspective for Perkinsus marinus. Journal of Shellfish Research 19: 642-643. (Abstract).
Bushek, D., C.F. Dungan and A.J. Lewitus. 2002a. Serological affinities of the oyster pathogen Perkinsus marinus (Apicomplexa) with some dinoflagellates (Dinophyceae). The Journal of Eukaryotic Microbiology 49: 11-16.
Bushek, D., S.E. Ford and M.M. Chintala. 2002b. Comparison of in vitro-cultured and wild type Perkinsus marinus. III. Fecal elimination and its role in transmission. Diseases of Aquatic Organisms 51: 217-225.
Bushek, D., J. Scarpa and S.E. Laramore. 2002c. Susceptibility of the Caribbean oyster Crassostrea rhizophorae to Perkinsus marinus. Journal of Shellfish Research 21: 371-372. (Abstract).
Bushek, D., D. Richardson, M.Y. Bobo and L.D. Coan. 2004. Quarantine of oyster shell cultch reduces the abundance of Perkinsus marinus. Journal of Shellfish Research 23: 369-373.
Bushek, D., M. Heidenreich and D. Porter. 2007. The effects of several common anthropogenic contaminants on proliferation of the parasitic oyster pathogen Perkinsus marinus. Marine Environmental Research 64: 535-540.
Butsic, E., R. Dame and D. Bushek. 2000. The effects of oyster removal on intensities of Perkinsus marinus infections in native oyster populations. Journal of Shellfish Research 19: 661. (Abstract).
Cáceres-Martínez, J., R. Vásquez-Yeomans, G. Padilla-Lardizábal and M.A. del Río Portilla. 2008. Perkinsus marinus in pleasure oyster Crassostrea corteziensis from Nayarit, Pacific coast of México. Journal of Invertebrate Pathology 99: 66-73.
Cáceres-Martínez, J., R. Vásquez-Yeomans and G. Padilla-Lardizábal. 2010. Parasites of the pleasure oyster Crassostrea corteziensis cultured in Nayarit, Mexico. Journal of Aquatic Animal Health 22: 141-151.
Cáceres-Martínez, J., M.G. Ortega, R. Vásquez-Yeomans, T.J.P. García, N.A. Stokes and R.B. Carnegie. 2012. Natural and cultured populations of the mangrove oyster Saccostrea palmula from Sinaloa, Mexico, infected by Perkinsus marinus. Journal of Invertebrate Pathology 110: 321-325.
Calvo, G.W. and E.M. Burreson. 1994. In vitro and in vivo effects of eight chemotherapeutants on the oyster parasite Perkinsus marinus (Mackin, Owen, and Collier). Journal of Shellfish Research 13: 101-107.
Calvo, G.W., M.W. Luckenbach, S.K. Allen Jr and E.M. Burreson. 1999. Comparative field study of Crassostrea gigas (Thunberg, 1793) and Crassostrea virginica (Gmelin 1791) in relation to salinity in Virginia. Journal of Shellfish Research 18: 465-473.
Calvo, G.W., M.W. Luckenbach and E.M. Burreson. 2000. High Performance of Crassostrea ariakensis in Chesapeake Bay. Journal of Shellfish Research 19: 643. (Abstract).
Calvo, G.W., M.W. Luckenbach, S.K. Allen Jr and E.M. Burreson. 2001. A comparative field study of Crassostrea ariakensis (Fujita 1913) and Crassostrea virginica (Gmelin 1791) in relation to salinity in Virginia. Journal of Shellfish Research 20: 221-229.
Carnegie, R. and E.M. Burreson. 2008. Factors contributing to the persistence of Crassostrea virginica populations in disease intense Virginia waters. Journal of Shellfish Research 27: 994. (Abstract).
Carnegie, R. and E.M. Burreson. 2011a. Impacts of Hematodinium nelsoni and Perkinsus marinus on gametogensis and spawning of Crassostrea virginica in Chesapeake Bay. Journal of Shellfish Research 30: 492. (Abstract).
Carnegie, R. and E.M. Burreson. 2011b. Evolutionary ecology of eastern oyster Crassostrea virginica and its parasites. Journal of Shellfish Research 30: 492. (Abstract).
Cheng, T.C. and J.J. Manzi. 1996. Correlation between the presence of lathyrose with the absence of Haplosporidium nelsoni in Crassostrea virginica from two South Carolina tributaries where Perkinsus marinus also inhibits hemocyte agglutination by the Lathyrus odoratus lectin. Journal of Shellfish Research 15: 391-394.
Chintala, M.M., S.E. Ford, W.S. Fisher and K.A. Ashton-Alcox. 1994. Oyster serum agglutinins and resistance to protozoan parasites. Journal of Shellfish Research 13: 115-121.
Chintala, M.M., D. Bushek and S.E. Ford. 2002. Comparison of in vitro-cultured and wild type Perkinsus marinus. II. Dosing methods and host response. Diseases of Aquatic Organisms 51: 203-216.
Choi, K.S., E.A. Wilson, D.H. Lewis, E.N. Powell and S.M. Ray. 1989. The energetic cost of Perkinsus marinus parasitism in oysters: quantification of the thioglycollate method. Journal of Shellfish Research 8: 125-131.
Choi, K., D.H. Lewis, E.N. Powell, P.F. Frelier and S.M. Ray. 1991. A polyclonal antibody developed from Perkinsus marinus hypnospores fails to cross react with other life stages of P. marinus in oyster (Crassostrea virginica) tissues. Journal of Shellfish Research 10: 411-415.
Chu, F.-L.E. 1988. Development and evaluation of techniques to study acquired immunity to Perkinsus marinus in the oyster, Crassostrea virginica (Gmelin). Journal of Shellfish Research 7: 51-55.
Chu, F.-L.E. 1996. Laboratory investigations of susceptibility, infectivity, and transmission of Perkinsus marinus in oysters. Journal of Shellfish Research 15: 57-66.
Chu, F.-L.E., A.K. Volety and G. Constatin. 1996. A comparison of Crassostrea gigas and Crassostrea virginica: effects of temperature and salinity on susceptibility to the protozoan parasite, Perkinsus marinus. Journal of Shellfish Research 15: 375-380.
Chu, F.-L.E. 1998. Host defenses against Perkinsus marinus: a review of recent findings in the eastern oyster, Crassostrea virginica. Journal of Shellfish Research 17: 321-322. (Abstract).
Chu, F.-L.E. 1999. Effects of temperature, salinity, and environmental pollutants on cellular and humoral responses in oysters (Crassostrea virginica). Journal of Shellfish Research 18: 321-322. (Abstract).
Chu, F.E. and K.H. Greene. 1989. Effect of temperature and salinity on in vitro culture of the oyster pathogen, Perkinsus marinus (Apicomplexa: Perkinsea). Journal of Invertebrate Pathology 53: 260-268.
Chu, F.-L.E. and R.C. Hale. 1994. Relationship between pollution and susceptibility to infectious disease in eastern oyster, Crassostrea virginica. Marine Environmental Research 38: 243-256.
Chu, F.-L.E. and J.F. La Peyre. 1989. Effect of environmental factors and parasitism on hemolymph lysozyme and protein of American oysters (Crassostrea virginica). Journal of Invertebrate Pathology 54: 224-232.
Chu, F.-L.E. and F. La Peyre. 1993. Perkinsus marinus susceptibility and defense-related activities in eastern oysters Crassostrea virginica: temperature effects. Diseases of Aquatic Organisms 16: 223-234.
Chu, F.L.E. and A.K. Volety. 1997a. The interaction of the oyster protozoan parasite, Perkinsus marinus and its host, the eastern oyster, Cassostrea virginica: a progress report. Journal of Shellfish Research 16: 261. (Abstract).
Chu, F.-L.E. and A.K. Volety. 1997b. Disease processes of the parasite Perkinsus marinus in eastern oyster Crassostrea virginica: minimum dose for infection initiation, and interaction of temperature, salinity and infective cell dose. Diseases of Aquatic Organisms 28: 61-68.
Chu, F.L.E., J.F. La Peyre and C.S. Burreson. 1993. Perkinsus marinus infection and potential defense-related activities in eastern oysters, Crassostrea virginica: salinity effects. Journal of Invertebrate Pathology 62: 226-232.
Chu, F.-L.E., Y. Huang, A.K. Volety and G. Constantin. 1998. Uptake, distribution and bioconversion of fluorescent lipid analogs in the oyster protozoan parasite, Perkinsus marinus. Journal of Shellfish Research 17: 322. (Abstract).
Chu, F.-L.E., P. Soudant, Y. Huang, A.K. Volety and G. Constantin. 1999. Uptake, distribution, and bioconversion of fluorescent lipid analogs in the oyster protozoan parasite, Perkinsus marinus. Journal of Shellfish Research 18: 318. (Abstract).
Chu, F.-L.E., E. Lund, P. Soudant and E. Harvey. 2002. De novo arachidonic acid synthesis in Perkinsus marinus, a protozoan parasite of the eastern oyster Crassostrea virginica. Molecular and Biochemical Parasitology 119: 179–190.
Chu, F.-L.E., P. Soudant and E.D. Lund. 2003.Perkinsus marinus, a protozoan parasite of the eastern oyster (Crassostrea virginica): effects of temperature on the uptake and metabolism of fluorescent lipid analogs and lipase activities. Experimental Parasitology 105: 121-130.
Chu, F.-L.E., E.D. Lund, E. Harvey and R. Adlof. 2004. Arachidonic acid synthetic pathways of the oyster protozoan parasite, Perkinsus marinus: evidence for usage of a delta-8 pathway. Molecular and Biochemical Parasitology 133: 45–51.
Chu, F.-L.E., E.D. Lund and J.A. Podbesek. 2008. Effects of triclosan on the oyster parasite, Perkinsus marinus and its host, the eastern oyster, Crassostrea virginica Journal of Shellfish Research 27: 769-773.
Coates, G.M., R.K. Cooper and J.F. La Peyre. 1999. Improvement of the whole-oyster procedure for enumerating Perkinsus marinus in oyster tissues. Journal of Shellfish Research 18: 328. (Abstract).
Cook, T., M. Folli, J. Klinck, S. Ford and J. Miller. 1998. The relationship between increasing sea-surface temperature and the northward spread of Perkinsus marinus (Dermo) disease epizootics in oysters. Estuarine, Coastal and Shelf Science 46: 587-597.
Coss, C.A., J.A.F. Robledo, G.M. Ruiz and G.R. Vasta. 2001. Description of Perkinsus andrewsi n.sp. isolated from the baltic clam (Macoma balthica) by characterization of the ribosomal RNA locus and development of a species-specific PCR-based diagnostic assay. The Journal of Eukaryotic Microbiology 48: 52-61.
Craig, A., E.N. Powell, R.R. Fay and J.M. Brooks. 1989. Distribution of Perkinsus marinus in Gulf coast oyster populations. Estuaries 12: 82-91.
Crosby, M.P. and C.F. Roberts. 1990. Seasonal infection intensity cycle of the parasite Perkinsus marinus (and an absence of Haplosporidium spp.) in oysters from a South Carolina salt marsh. Diseases of Aquatic Organisms 9: 149-155.
Culbertson, J., J. Anderson and S. Ray. 2011. Monitoring Dermo infections in Texas oyster populations and their response to changes in freshwater inflows. Journal of Shellfish Research 30: 498. (Abstract).
da Silva, P.M., R.T. Vianna, R.C. Sabry, A.R.M. Magalhães, G. Boehs, M.P. Scardua, C. Guertler, L.P. Ferreira, R.P. Brandâo, L.N. Santana, A. Villalba, S. Fernández, A. Ramilo, A. Cao, K. Reece, C. Dungan and M.A. Barracco. 2012. Status of Perkinsus spp. in oysters Crassostrea rhizophorae and C. brasiliana from Brazil: first report of P. marinus. Journal of Shellfish Research 31: 346. (Abstract).
da Silva, P.M., R.T. Vianna, C. Guertler, L.P. Ferreira, L.N. Santana, S. Fernández-Boo, A. Ramilo, A. Cao and A. Villalba. 2013. First report of the protozoan parasite Perkinsus marinus in South America, infecting mangrove oysters Crassostrea rhizophorae from the Paraíba River (NE, Brazil). Journal of Invertebrate Pathology 113: 96-103.
De Faveri, J., R.M. Smolowitz and S.B. Roberts. 2009. Development and validation of a real-time quantitative PCR assay for the detection and quantification of Perkinsus marinus in the eastern oyster, Crassostrea virginica. Journal of Shellfish Research 28: 459-464.
Dégremont, L., C. Garcia, A. Frank-Lawale and S.K. Allen Jr. 2012. Triploid oysters in the Chesapeake Bay: comparison of diploid and triploid Crassostrea virginica. Journal of Shellfish Research 31: 21-31.
Delaney, M.A., Y.J. Brady, S.D. Worley and K.L. Huels. 2003. The effectiveness of N-halamine disinfectant compounds on Perkinsus marinus, a parasite of the eastern oyster Crassostrea virginica. Journal of Shellfish Research 22: 91-94.
Dittman, D.E., S.E. Ford and D.K. Padilla. 2001. Effects of Perkinsus marinus on reproduction and condition of the eastern oyster, Crassostrea virginica, depending on timing. Journal of Shellfish Research 20: 1025-1034.
Dungan, C.F. 1997. Perkinsus marinus: immunoassay detection in oyster tissues and environmental samples and in vitro experimental systems. Journal of Shellfish Research 16: 263. (Abstract).
Dungan, C.F. and R.M. Hamilton. 1995. Use of a tetrazolium-based cell proliferation assay to measure effects of in vitro conditions on Perkinsus marinus (Apicomplexa) proliferation. The Journal of Eukaryotic Microbiology 42: 379-388.
Dungan, C.F. and R.M. Hamilton. 1997. Microplate ELISA assay for detection of Perkinsus marinus in oyster tissues. Journal of Shellfish Research 16: 330-331. (Abstract).
Dungan, C.F. and B.S. Roberson. 1993. Binding specificities of mono- and polyclonal antibodies to the protozoan oyster pathogen Perkinsus marinus. Diseases of Aquatic Organisms 15: 9-22.
Dungan, C.F., K.S. Reece, R.M. Hamilton, N.A. Stokes and E.M. Burreson. 2007. Experimental cross-infection by Perkinsus marinus and P. chesapeaki in three sympatric species of Chesapeake Bay oysters and clams. Diseases of Aquatic Organisms 76: 67-75.
Dungan, C.F., R.B. Carnegie, K.M. Hill, C.B. McCollough, S.E. Laramore, C.J. Kelly, N.A. Stokes and J. Scarpa. 2012. Diseases of oysters Crassostrea ariakensis and C. virginica reared in ambient waters from the Choptank River, Maryland and the Indian River Lagoon, Florida. Diseases of Aquatic Organisms 101: 173-183.
Earnhart, C.G., M.A. Vogelbein, G.D. Brown, K.S. Reece and S.L. Kaattari. 2004. Supplementation of Perkinsus marinus cultures with host plasma or tissue homogenate enhances their infectivity. Applied and Environmental Microbiology 70: 421-431.
Elandalloussi, L.M., R.M. Leite, R. Afonso, P.A. Nunes, J.A.F. Robledo, G.R. Vasta and M.L. Cancela. 2004. Development of a PCR-ELISA assay for diagnosis of Perkinsus marinus and Perkinsus atlanticus infections in bivalve molluscs. Molecular and Cellular Probes 18: 89-96.
Ellin, R.C. and D. Bushek. 1999. Potential use of Ray's fluid thioglycollate medium to detect and quantify Perkinsus marinus in environmental water samples. Journal of Shellfish Research 18: 328. (Abstract).
Ellin, R. and D. Bushek. 2000. An examination of ecological factors governing planktonic abundance and dispersal of Perkinsus marinus. Journal of Shellfish Research 19: 644. (Abstract).
Ellin, R. and D. Bushek. 2006. Adaptation of Ray's fluid thioglycolate medium assay to detect and quantify planktonic stages of Perkinsus spp. parasites. Journal of Shellfish Research 25: 1037-1042.
Elsayed, E. and M. Faisal. 1999. Correlation between the level of protease inhibitors and intensity of Perkinsus marinus infection in eastern oyster (Crassostrea virginica). Journal of Shellfish Research 18: 328. (Abstract).
Encomio, V.G. and F.-L.E. Chu. 2005. Season variation of heat shock protein 70 in eastern oysters (Crassostrea virginica) infected with Perkinsus marinus (dermo). Journal of Shellfish Research 24: 167-175.
Encomio, V.G. and F.L.E. Chu. 2007. Heat shock protein (hsp70) expression and thermal tolerance in sublethally heat-shocked eastern oysters Crassostrea virginica infected with the parasite Perkinsus marinus. Diseases of Aquatic Organisms 76: 251-260.
Encomio, V.G., S.M. Stickler, S.K. Allen Jr and F.-L. Chu. 2005. Performance of "natural dermo-resistant" oyster stocks - survival, disease, growth, condition and energy reserves. Journal of Shellfish Research 24: 143-155.
Enríquez-Espinoza, T.L., J.M. Grijalva-Chon, R. Castro-Longoria and J. Ramos-Paredes. 2010. Perkinsus marinus in Crassostrea gigas in the Gulf of California. Diseases of Aquatic Organisms 89: 269-273.
Espinosa, E.P., S. Winnicki and B. Allam. 2013. Early host-pathogen interactions in a marine bivalve: Crassostrea virginica pallial mucus modulates Perkinsus marinus growth and virulence. Diseases of Aquatic Organisms 104: 237-247.
Faisal, M. 1999. The role of protease-antiprotease interactions in Perkinsus marinus infection in Crassostrea spp. Journal of Shellfish Research 18: 322. (Abstract).
Faisal, M., J.F. La Peyre and S.L. Kaattari. 1997. A promising chemotherapy for Perkinsus marinus-infected oysters. Journal of Shellfish Research 16: 263-264. (Abstract).
Faisal, M., J.F. La Peyer, E. Elsayed and D.C. Wright. 1999. Bacitracin inhibits the oyster pathogen Perkinsus marinus in vitro and in vivo. Journal of Aquatic Animal Health 11: 130-138.
Feng, C., C. Wang, X. Lin, Y. Zhang, J. Lv, J.-h. Deng, X. Yuan, L. Mei and S.-q. Wu. 2013. Development of a loop-mediated isothermal amplification method for detection of Perkinsus spp. in mollusks. Diseases of Aquatic Organisms 104: 141-148.
Fernández-Robledo, J.A., E.J. Schott and G.R. Vasta. 2008a. Perkinsus marinus superoxide dismutase 2 (PmSOD2) localizes to single-membrane subcellular compartments. Biochemical and Biophysical Research Communications 375: 215-219.
Fernández-Robledo, J.A., Z. Lin and G.R. Vasta. 2008b. Transfection of the protozoan parasite Perkinsus marinus. Molecular and Biochemical Parasitology 157: 44-53.
Fisher, W.S. and L.M. Oliver. 1996. A whole-oyster procedure for diagnosis of Perkinsus marinus disease using Ray's fluid thioglycollate culture medium. Journal of Shellfish Research 15: 109-117.
Fisher, W.S., J.D. Gauthier and J.T. Winstead. 1992. Infection intensity of Perkinsus marinus disease in Crassostrea virginica (Gmelin, 1791) from the Gulf of Mexico maintained under different laboratory conditions. Journal of Shellfish Research 11: 363-369.
Fong, D., R. Rodriguez, K. Koo, J. Sun, M.L. Sogin, D. Bushek, D.T.J. Littlewood and S.E. Ford. 1993. Small subunit ribosomal RNA gene sequence of the oyster parasite Perkinsus marinus. Molecular Marine Biology and Biotechnology 2: 346-350.
Ford, S.E. 1992. Avoiding the transmission of disease in commercial culture of molluscs, with special reference to Perkinsus marinus (Dermo) and Haplosporidium nelsoni (MSX). Journal of Shellfish Research 11: 539-546.
Ford, S.E. 1996. Range extension by the oyster parasite Perkinsus marinus into the northeastern United States: response to climate change? Journal of Shellfish Research 15: 45-56.
Ford, S.E. and M.M. Chintala. 2006. Northward expansion of a marine parasite: Testing the role of temperature adaptation. Journal of Experimental Marine Biology and Ecology 339: 226–235.
Ford, S.E. and R. Smolowitz. 2007. Infection dynamics of an oyster parasite in its newly expanded range. Marine Biology 151: 119–133.
Ford, S.E. and M.R. Tripp. 1996. Diseases and Defense Mechanisms. In: Kennedy, V.S., R.I.E. Newell, A.F. Eble (eds.) The Eastern Oyster Crassostrea virginica. Maryland Sea Grant College, College Park, Maryland. pp. 581-660.
Ford, S.E., A. Schotthoefer and C. Spruck. 1999. In vivo dynamics of the microparasite Perkinsus marinus during progression and regression of infections in eastern oysters. The Journal of Parasitology 85: 273-282.
Ford, S., R. Smolowitz and M. Chintala. 2000a. The question of temperature and Perkinsus marinus (Dermo) activity in the northeastern United States. Journal of Shellfish Research 19: 571. (Abstract).
Ford, S.E., R. Smolowitz and M.M. Chintala. 2000b. Temperature and range extension by Perkinsus marinus. Journal of Shellfish Research 19: 598. (Abstract).
Ford, S., Z. Xu and G. DeBrosse. 2000c. Acquisition and prevention of MSX and Dermo in a hatchery and land-based nursery: a DNA assay investigation. Journal of Shellfish Research 19: 571. (Abstract).
Ford, S.E., Z. Xu and G. Debrosse. 2001. Use of particle filtration and UV irradiation to prevent infection by Haplosporidium nelsoni (MSX) and Perkinsus marinus (Dermo) in hatchery-reared larval and juvenile oysters. Aquaculture 194: 37-49.
Ford, S.E., M.M. Chintala and D. Bushek. 2002. Comparison of in vitro-cultured and wild type Perkinsus marinus. I. Pathogen virulence. Diseases of Aquatic Organisms 51: 187-201.
Foster, B., S. Grewal, O. Graves, F.M. Hughes Jr and I.M. Sokolova. 2011. Copper exposure affects hemocyte apoptosis and Perkinsus marinus infection in eastern oysters Crassostrea virginica (Gmelin). Fish and Shellfish Immunology 31: 341-349.
Gaffney, P.M. and D. Bushek. 1996. Genetic aspects of disease resistance in oysters. Journal of Shellfish Research 15: 135-140.
Garreis, K.A., J.F. La Peyre and M. Faisal. 1996. The effects of Perkinsus marinus extracellular products and purified proteases on oyster defence parameters in vitro. Fish and Shellfish Immunology 6: 581-597.
Gauthier, J.D. and W.S. Fisher. 1990. Hemolymph assay for diagnosis of Perkinsus marinus in oysters Crassostrea virginica (Gmelin, 1791). Journal of Shellfish Research 9: 367-371.
Gauthier, J.D. and G.R. Vasta. 1993. Continuous in vitro culture of the eastern oyster parasite Perkinsus marinus. Journal of Invertebrate Pathology 62: 321-323.
Gauthier, D.J. and G.R. Vasta. 1994. Inhibition of in vitro replication of the oyster parasite Perkinsus marinus by the natural iron chelators transferrin, lactoferrin, and desferrioxamine. Developmental and Comparative Immunology 18: 277-286.
Gauthier, J.D. and G.R. Vasta. 1995. In vitro culture of the eastern oyster parasite Perkinsus marinus: optimization of the methodology. Journal of Invertebrate Pathology 66: 156-168.
Gauthier, J.D. and G.R. Vasta. 2002. Effects of plasma from bivalve mollusk species on the in vitro proliferation of the protistan parasite Perkinsus marinus. Journal of Experimental Zoology 292: 221-230.
Gauthier, J.D., T.M. Soniat and J.S. Rogers. 1990. A parasitological survey of oysters along salinity gradients in coastal Louisiana. Journal of the World Aquaculture Society 21: 105-115.
Gauthier, J.D., B. Feig and G.R. Vasta. 1995. Effect of fetal bovine serum glycoproteins on the in vitro proliferation of the oyster parasite Perkinsus marinus: development of a fully defined medium. The Journal of Eukaryotic Microbiology 42: 307-313.
Gauthier, J.D., C.R. Miller and A.E. Wilbur. 2006. TaqMan® MGB real-time PCR approach to quantification of Perkinsus marinus and Perkinsus spp. in oysters Journal of Shellfish Research 25: 619-624. (for Erratum see J. Shellfish Res. 25(3): 1105).
Goedken, M., B. Morsey, I. Sunila and S. de Guise. 2005. Immunomodulation of Crassostrea gigas and Crassostrea virginica cellular defense mechanisms by Perkinsus marinus. Journal of Shellfish Research 24: 487-496.
Goggin, C.L. 1994. Variation in the two internal transcribed spacers and 5.8S ribosomal RNA from five isolates of the marine parasite Perkinsus (Protista, Apicomplexa). Molecular and Biochemical Parasitology 65: 179-182.
Goggin, C.L. and S.C. Barker. 1993. Phylogenetic position of the genus Perkinsus (Protista, Apicomplexa) based on small subunit ribosomal RNA. Molecular and Biochemical Parasitology 60: 65-70.
Goulletquer, P., M. Héral and B.J. Rothschild. 1994. Causes of decline of oyster production (Crassostrea virginica) in the Maryland portion of the Chesapeake Bay: a literature study. Haliotis 23: 87-112. Open Access version: https://archimer.ifremer.fr/doc/00000/3081/. (in French only)
Green-Beach, E., X. Guo, P. Smouse and D. Bushek. 2011. Population structure of oysters on Martha's Vineyard, MA in response to selection by Perkinsus marinus, using microsatellite markers. Journal of Shellfish Research 30: 512. (Abstract).
Grizel, H. 1987. Les maladies des mollusques: étiologie et progrès récents des recherches. Oceanis 13: 357-370.
Gullian-Klanian, M., J.A. Herrera-Silveira, R. Rodríguez-Canul and L. Aguirre-Macedo. 2008. Factors associated with the prevalence of Perkinsus marinus in Crassostrea virginica from the southern Gulf of Mexico. Diseases of Aquatic Organisms 39: 237–247.
He, Y., H. Yu, Z. Bao, Q. Zhang and X. Guo. 2012. Mutation in promoter region of a serine protease inhibitor confers Perkinsus marinus resistance in the eastern oyster (Crassostrea virginica). Fish and Shellfish Immunology 33: 411-417.
Hearne, J.L. and J.S. Pitula. 2011. Identification of two spliced leader RNA transcripts from Perkinsus marinus. The Journal of Eukaryotic Microbiology 58: 266-268.
Héral, M., B.J. Rothschild and P. Goulletquer. 1990. Decline of oyster production in the Maryland portion of the Chesapeake Bay: causes and perspectives. International Council for Exploration of the Sea C.M.1990/K:20: 38 pp. Open Access version: https://archimer.ifremer.fr/doc/00000/2393/. (in French only)
Hoese, H.D. 1964. Studies on oyster scavengers and their relation to the fungus Dermocystidium marinum. Proceedings of the National Shellfisheries Association 53: 161-174.
Hofmann, E.E., E.N. Powell, J.M. Klinck and G. Saunders. 1995. Modelling diseased oyster populations: I. modelling Perkinsus marinus infection in oysters. Journal of Shellfish Research 14: 121-151.
Hofmann, E.E., J.M. Klinck, S.E. Ford and E.N. Powell. 1999. Disease dynamics: modeling the effect of climate change on oyster disease. Journal of Shellfish Research 18: 329. (Abstract).
ICES. 2004. Trends in important diseases affecting fish and molluscs in the ICES area 1998-2002. International Council for the Exploration of the Sea, Copenhagen, Denmark. (Prepared and edited by the Working Group on Pathology and Diseases of Marine Organisms.).
Jordan, S.J. 1995. Population and disease dynamics of Maryland oyster bars: a multivariate classification analysis. Journal of Shellfish Research 14: 459-468.
Jordan, P.J. and L.E. Deaton. 2005. Characterization of phenoloxidase from Crassostrea virginica hemocytes and the effect of Perkinsus marinus on phenoloxidase activity in the hemolymph of Crassostrea virginica and Geukensia demissa. Journal of Shellfish Research 24: 477-482.
Joseph, S.J., J.A. Fernández-Robledo, M.J. Gardner, N.M. El-Sayed, C.H. Kuo, E.J. Schott, H. Wang, J.C. Kissinger and G.R. Vasta. 2010. The alveolate Perkinsus marinus: biological insights from EST gene discovery. BMC Genomics 11: 228 (open access see https://www.biomedcentral.com/1471-2164/11/228).
Kaattari, S.L., D.A. Shapiro, T.D. Lewis and M. Faisal. 1997. Development of enhanced diagnostics and identification of oyster target molecules for Perkinsus marinus. Journal of Shellfish Research 16: 266. (Abstract).
Karolus, J., I. Sunila, S. Spear, J. DeCrescenzo and J. Volk. 1999. The presence of Haplosporidium nelsoni (MSX) and Perkinsus marinus (Dermo) in Crassostrea virginica along the Connecticut and northern Long Island shoreline in 1998 - an extensive survey. Journal of Shellfish Research 18: 270. (Abstract).
Karolus, J., I. Sunila, S. Spear and J. Volk. 2000. Prevalence of Perkinsus marinus (Dermo) in Crassostrea virginica along the Connecticut shoreline. Aquaculture 183: 215–221.
Kennedy, V.S., R.I.E. Newell, G.E. Krantz and S. Otto. 1995. Reproductive capacity of the eastern oyster Crassostrea virginica infected with the parasite Perkinsus marinus. Diseases of Aquatic Organisms 23: 135-144.
Kern, F.G. 1985. Perkinsus marinus parasitism, a sporozoan disease of oyster. In: Sindermann, C.J. (ed.) Identification Leaflets for Diseases and Parasites of Fish and Shellfish. International Council for the Eploration of the Sea, Copenhagen. pp. 1-4.
Kern, F.G., L.C. Sullivan and M. Takata. 1973. Labyrinthomyxa-like organisms associated with mass mortalities of oysters Crassostrea virginica, from Hawaii. Proceedings of the National Shellfisheries Association 63: 43-46.
Kim, Y. and E.N. Powell. 1998. Influence of climate change on interannual variation in population attributes of Gulf of Mexico oysters. Journal of Shellfish Research 17: 265-274.
Kim, Y., K.A. Ashton-Alcox, and E.N. Powell. 2006. Histological Techniques for Marine Bivalve Molluscs: Update. Silver Spring, MD. NOAA Technical Memorandum NOS NCCOS 27. pp. 53-74.
Kingsley-Smith, P.R., H.D. Harwell, M.L. Kellogg, S.M. Allen, S.K. Allen Jr, D.W. Meritt, K.T. Paynter Jr and M.W.
Luckenbach. 2009. Survival and growth of triploid Crassostrea virginica (Gmelin, 1791) and C. ariakensis (Fujita, 1913) in bottom environments of Chesapeake Bay: implications for an introduction. Journal of Shellfish Research 28: 169-184.
Kleinschuster, S.J. and J. Parent. 1995. Sub-clinical infection of oysters (Crassostrea virginica) (Gmelin 1791) from Maine by species of the genus Perkinsus (Apicomplexa). Journal of Shellfish Research 14: 489-491.
Kleinschuster, S.J. and S.L. Swink. 1993. A simple method for the in vitro culture of Perkinsus marinus. The Nautilus 107: 76-78.
Klinck, J.M., E.N. Powell, J.N. Kraeuter, S.E. Ford and K.A. Ashton-Alcox. 2001. A fisheries model for managing the oyster fishery during times of disease. Journal of Shellfish Research 20: 977-989.
Krantz, G.E. 1994. Chemical inhibition of Perkinsus marinus in two in vitro culture systems. Journal of Shellfish Research 13: 131-136.
Krantz, G.E. and S.J. Jordan. 1996. Management alternatives for protecting Crassostrea virginica fisheries in Perkinsus marinus enzootics and epizootic areas. Journal of Shellfish Research 15: 167-176.
La Peyre, J.F. 1996. Propogation and in vitro studies of Perkinsus marinus. Journal of Shellfish Research 15: 89-101.
La Peyre, J.F. and F.-L.E. Chu. 1994. A simple procedure for the isolation of Perkinsus marinus merozoites, a pathogen of the eastern oyster, Crassostrea virginica. Bulletin of the European Association of Fish Pathologists 14: 101-103.
La Peyre, J.F. and R.K. Cooper. 1998. Virulence factors of Perkinsus marinus: recent findings and questions. Journal of Shellfish Research 17: 330-331. (Abstract).
La Peyre, J.F. and M. Faisal. 1995a. Improved method for the initiation of continuous cultures of the oyster pathogen Perkinsus marinus (Apicomplexa). Transactions of the American Fisheries Society 124: 144-146.
La Peyer, J.F. and M. Faisal. 1995b. Perkinsus marinus produces extracellular proteolytic factor(s) in vitro. Bulletin of the European Association of Fish Pathologists 15: 28-31.
La Peyer, J.F. and M. Faisal. 1996. Optimal culture conditions for the propagation of the oyster pathogen Perkinsus marinus (Apicomplexa) in protein deficient medium. Parasite 3: 147-153.
La Peyre, J.F. and M. Faisal. 1997a. Extracellular proteins of the oyster pathogen Perkinsus marinus as virulence factors and potential targets for chemotherapy. Journal of Shellfish Research 16: 269. (Abstract).
La Peyre, J.F. and M. Faisal. 1997b. Development of a protein-free chemically defined culture medium for the propagation of the oyster pathogen Perkinsus marinus. Parasite 4: 67-73.
La Peyre, J.F. and A.K. Volety. 1999. Modulation of eastern oyster hemocyte activity by Perkinsus marinus extracellular proteins. Journal of Shellfish Research 18: 322. (Abstract).
La Peyre, J.F., M. Faisal and E.M. Burreson. 1993. In vitro propagation of the protozoan Perkinsus marinus, a pathogen of the eastern oyster, Crassostrea virginica. The Journal of Eukaryotic Microbiology 40: 304-310.
La Peyre, J.F., F.L.E. Chu and J.M. Meyers. 1995a. Haemocytic and humoral activities of eastern and Pacific oysters following challenge by the protozoan Perkinsus marinus. Fish and Shellfish Immunology 5: 179-190.
La Peyre, J.F., F.-L.E. Chu and W.K. Vogelbein. 1995b. In vitro interaction of Perkinsus marinus merozoites with Eastern and Pacific oyster hemocytes. Developmental and Comparative Immunology 19: 291-304.
La Peyre, M.K., A.D. Nickens, A.K. Volety, G.S. Tolley and J.F. La Peyer. 2003. Environmental significance of freshets in reducing Perkinsus marinus infection in eastern oysters Crassostrea virginica: potential management applications. Marine Ecology Progress Series 248: 165-176.
La Peyre, M., S. Casas and J. La Peyre. 2006. Salinity effects on viability, metabolic activity and proliferation of three Perkinsus species. Diseases of Aquatic Organisms 71: 59-74.
La Peyre, J., S. Casas, Y. Li and J. Supan. 2008a. Evaluation of an adjustable long line system to increase oyster survival in Dermo endemic areas and refrigerated shelf-life after harvest. Journal of Shellfish Research 27: 1041.
La Peyre, M.K., S.M. Casas, A. Villalba and J.F. La Peyre. 2008b. Determination of the effects of temperature on viability, metabolic activity and proliferation of two Perkinsus species, and its significance to understanding seasonal cycles of perkinsosis. Parasitology 135: 505-519.
La Peyre, M.K., B. Gossman and J.F. La Peyre. 2009. Defining optimal freshwater flow for oyster production: effects of freshet rate and magnitude of change and duration on eastern oysters and Perkinsus marinus infection. Estuaries and Coasts 32: 522-534.
La Peyre, J.F., Q.-G. Xue, N. Itoh, Y. Li and R.K. Cooper. 2010a. Serine protease inhibitor cvSI-1 potential role in the eastern oyster host defense against the protozoan parasite Perkinsus marinus. Developmental and Comparative Immunology 34: 84-92.
La Peyre, M.K., S.M. Casas, W. Gayle and J.F. La Peyre. 2010b. The combined influence of sub-optimal temperature and salinity on the in vitro viability of Perkinsus marinus, a protistan parasite of the eastern oyster Crassostrea virginica. Journal of Invertebrate Pathology 105: 176-181.
Lenihan, H.S., F. Micheli, S.W. Shelton and C.H. Peterson. 1999. The influence of multiple environmental stressors on susceptibility to parasites: an experimental determination with oysters. Limnology and Oceanography 44: 910-924.
Leonard III, K., M. Gomez-Chiarri and A. Ganz. 1999. Detecting the presence of Perkinsus marinus in the eastern oyster, Crassostrea virginica in Rhode Island waters. Journal of Shellfish Research 18: 331. (Abstract).
Leonard III, K., J. Dougal and M. Gomez-Chiarri. 2000. Detecting the presence of Perkinsus marinus and Haplosporidium nelsoni in the oyster, Crassostrea virginica, in Rhode Island waters: a survey update. Journal of Shellfish Research 19: 575. (Abstract).
Levine, N.D. 1978. Perkinsus gen. n. and other new taxa in the protozoan phylum Apicomplexa. The Journal of Parasitology 64: 549.
Lewis, E.J., F.G. Fern, A. Rosenfield, S.A. Stevens, R.L. Walker and P.B. Heffernan. 1992. Lethal parasites in oysters from coastal Georgia with discussion of disease and management implications. Marine Fisheries Review 54: 1-6.
Lund, E.D. and F.-L.E. Chu. 2002. Phospholipid biosynthesis in the oyster protozoan parasite, Perkinsus marinus. Molecular and Biochemical Parasitology 121: 245-253.
Lund, E.D., F.-L.E. Chu and E. Harvey. 2004. In vitro effects of temperature and salinity on fatty acid synthesis in the oyster protozoan parasite Perkinsus marinus. Journal of Experimental Marine Biology and Ecology 307: 111-126.
Lund, E.D., P. Soudant, F.-L.E. Chu, E. Harvey, S. Bolton and A. Flowers. 2005. Effects of triclosan on growth, viability and fatty acid synthesis of the oyster protozoan parasite Perkinsus marinus. Diseases of Aquatic Organisms 67: 217–224.
Lund, E.D., F.-L.E. Chu, P. Soudant and E. Harvey. 2007. Perkinsus marinus, a protozoan parasite of the eastern oyster, has a requirement for dietary sterols. Comparative Biochemistry and Physiology Part A146: 141-147.
MacIntyre, E.A., C.G. Earnhart and S.L. Kaattari. 2003. Host oyster tissue extracts modulate in vitro protease expression and cellular differentiation in the protozoan parasite, Perkinsus marinus. Parasitology 126: 293-302.
Mackin, J.G. 1951. Histopathology of infection of Crassostrea virginica (Gmelin) by Dermocystidium marinum Mackin, Owen, and Collier. Bulletin of Marine Science of the Gulf and Caribbean 1: 72-87.
Mackin, J.G. 1955. Dermocystidium marinum and salinity. Proceedings of the National Shellfisheries Association 46: 116-128.
Mackin, J.G. and S.M. Ray. 1966. The taxonomic relationship of Dermocystidium marinum Mackin, Owen, and Collier. Journal of Invertebrate Pathology 8: 544-545.
Mackin, J.G., H.M. Owen and A. Collier. 1950. Preliminary note on the occurrence of a new protistan parasite, Dermocystidium marinum n.sp. in Crassostrea virginica (Gmelin). Science (Washington D C) 111: 328-329.
Marsh, A.G., J.D. Gauthier and G.R. Vasta. 1995. A semiquantitative PCR assay for assessing Perkinsus marinus infections in the eastern oyster, Crassostrea virginica. The Journal of Parasitology 81: 577-583.
Mangot, J.F., D. Debroas and I. Domaizon. 2011. Perkinsozoa, a well-known marine protozoan flagellate parasite group, newly identified in lacustrine systems: a review. Hydrobiologia 659: 37-48.
Matsuzaki, M., H. Kuroiwa, T. Kuroiwa, K. Kita and H. Nozaki. 2008. A cryptic algal group unveiled: a plastid biosynthesis pathway in the oyster parasite Perkinsus marinus. Molecular Biology and Evolution 25: 1167-1179.
McCollough, C.B., B.W. Albright, G.R. Abbe, L.S. Barker and C.F. Dungan. 2007. Acquisition and progression of Perkinsus marinus infections by specific-pathogen-free juvenile oysters (Crassostrea virginica Gmelin) in a mesohaline Chesapeake Bay tributary. Journal of Shellfish Research 26: 465-477.
McCoy, A., S.M. Baker and A.C. Wright. 2007. Investigation of Perkinsus spp. in aquacultured hard clams (Mercenaria mercenaria) from the Florida Gulf coast. Journal of Shellfish Research 26: 1029-1033.
Meyers, J.A., E.M. Burreson, B.J. Barber and R. Mann. 1991. Susceptibility of diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg, 1793) and eastern oysters, Crassostrea virginica (Gmelin, 1791), to Perkinsus marinus. Journal of Shellfish Research 10: 433-437.
Mortensen, S., I. Arzul, L. Miossec, C. Paillard, S. Feist, G. Stentiford, T. Renault, D. Saulnier and A. Gregory. 2007. Molluscs and crustaceans, 5.3.19 Perkinsosis due to Perkinsus marinus. In: Raynard, R., T. Wahli, I. Vatsos, S. Mortensen (eds.) Review of disease interactions and pathogen exchange between farmed and wild finfish and shellfish in Europe. VESO on behalf of DIPNET, Oslo. p. 397-407.
Moss, J.A., E.M. Burreson and K.S. Reece. 2006. Advanced Perkinsus marinus infections in Crassostrea ariakensis maintained under laboratory conditions. Journal of Shellfish Research 25: 65-72.
Muñoz, P., K. Vance and M. Gómez-Chiarri. 2003. Protease activity in the plasma of American oysters, Crassostrea virginica, experimentally infected with the protozoan parasite Perkinsus marinus. The Journal of Parasitology 89: 941-951.
Nickens, A.D., T.R. Tiersch and J.F. La Peyre. 2000a. Effect of a lytic peptide and protease inhibitors on Perkinsus marinus in infected hemocytes of eastern oysters. Journal of Shellfish Research 19: 647. (Abstract).
Nickens, A.D., E. Wagner and J.F. La Peyer. 2000b. Improved procedure to count Perkinsus marinus in eastern oyster hemolymph. Journal of Shellfish Research 19: 665. (Abstract).
Nickens, A.D., J.F. La Peyre, E.S. Wagner and T.R. Tiersch. 2002. An improved procedure to count Perkinsus marinus in eastern oyster hemolymph. Journal of Shellfish Research 21: 725-732.
Norén, F., O. Moestrup and A.-S. Rehnstam-Holm. 1999. Parvilucifera infectans Norén et Moestrup gen. et sp. nov. (Perkinsozoa phylum nov.): a parasitic flagellate capable of killing toxic microalgae. European Journal of Protistology 35: 233-245.
Norton, J.H., M.A. Shepherd, F.O. Perkins and H.C. Prior. 1993. Perkinsus-like infection in farmed golden-lipped pearl oyster Pinctada maxima from the Torres Strait, Australia. Journal of Invertebrate Pathology 62: 105-106.
O'Farrell, C.L., J.F. La Peyre, K.T. Paynter and E.M. Burreson. 2000. Osmotic tolerance and volume regulation in in vitro cultures of the oyster pathogen Perkinsus marinus. Journal of Shellfish Research 19: 139-145.
Oliver, J.L., M. Faisal and S.L. Kaattari. 1998a. The effect of oyster hemolytic factors on Perkinsus marinus. Journal of Shellfish Research 17: 334-335. (Abstract).
Oliver, L.M., W.S. Fisher, S.E. Ford, L.M. Ragone Calvo, E.M. Burreson, E.B. Sutton and J. Gandy. 1998b. Perkinsus marinus tissue distribution and seasonal variation in oysters Crassostrea virginica from Florida, Virginia and New York. Diseases of Aquatic Organisms 34: 51-61.
Oliver, J.L., M. Faisal and S.L. Kaattari. 1999a. Plasma of Crassostrea spp. possess a low molecular weight inhibitor of Perkinsus marinus protease. Journal of Shellfish Research 18: 319. (Abstract).
Oliver, J.L., T.D. Lewis, M. Faisal and S.L. Kaattari. 1999b. Analysis of the effects of Perkinsus marinus proteases on plasma proteins of the eastern oyster (Crassostrea virginica) and the Pacific oyster (Crassostrea gigas). Journal of Invertebrate Pathology 74: 173–183.
Oliver, J.L., P.M. Gaffney, S.K. Allen Jr, M. Faisal and S.L. Kaattari. 2000. Protease inhibitory activity in selectively bred families of eastern oysters. Journal of Aquatic Animal Health 12: 136-145.
Ottinger, C.A., T.D. Lewis, D.A. Shapiro, M. Faisal and S.L. Kaattari. 2001. Detection of Perkinsus marinus extracellular proteins in tissues of the eastern oyster Crassostrea virginica: potential use in diagnostic assays. Journal of Aquatic Animal Health 13: 133-141.
Panko, C., V. Encomio, J. Barreto and A.K. Volety. 2008. In vitro and in vivo evaluation of quinine as a potential anti-protozoal for the eastern oyster parasite Perkinsus marinus. Journal of Shellfish Research 27: 789-793.
Paynter, K.T. 1996. The effects of Perkinsus marinus infection on physiological processes in the eastern oyster, Crassostrea virginica. Journal of Shellfish Research 15: 119-125.
Paynter, K.T. and E.M. Burreson. 1991. Effects of Perkinsus marinus infection in the eastern oyster, Crassostrea virginica: II. Disease development and impact on growth rate at different salinities. Journal of Shellfish Research 10: 425-431.
Paynter, K.T., C. Caudill and E.M. Burreson. 1993a. The physiological effects of protozoan parasitism on the eastern oyster, Crassostrea virginica: introductory overview. Journal of Shellfish Research 12: 137. (Abstract).
Paynter, K.T., S.K. Pierce and E.M. Burreson. 1993b. The physiological effects of protozoan parasitism on the eastern oyster Crassostrea virginica: effects on cellular free amino acid levels. Journal of Shellfish Research 12: 137. (Abstract).
Paynter, K.T., S.K. Pierce and E.M. Burreson. 1995. Level of intracellular free amino acids used for salinity tolerance by oysters (Crassostrea virginica) are altered by protozoan (Perkinsus marinus) parasitism. Marine Biology 122: 67-72.
Paynter, K.T., J.D. Goodwin, M.E. Chen, N.J. Ward, M.W. Sherman, D.W. Meritt and S.K. Allen. 2008. Crassostrea ariakensis in Chesapeake Bay: growth, disease and mortality in shallow subtidal environments. Journal of Shellfish Research 27: 509-515.
Paynter, K.T., V. Politano, H.A. Lane, S.M. Allen and D. Meritt. 2010. Growth rates and prevalence of Perkinsus marinus in restored oyster populations in Maryland. Journal of Shellfish Research 29: 309-317.
Pecher, W.T., M.R. Alavi, E.J. Schott, J.A. Fernandez-Robledo, L. Roth, S.T. Berg and G.R. Vasta. 2008. Assessment of the northern distribution range of selected Perkinsus species in eastern oysters (Crassostrea virginica) and hard clams (Mercenaria mercenaria) with the use of PCR-based detection assays. The Journal of Parasitology 95: 410–422.
Penna, S., R.A. French, J. Volk, J. Karolus, I. Sunila and R. Smolowitz. 1999. Diagnostic screening of oyster pathogens: preliminary field trials of multiplex PCR. Journal of Shellfish Research 18: 319-320. (Abstract).
Penna, M.-S., M. Khan and R.A. French. 2001. Development of a multiplex PCR for the detection of Haplosporidium nelsoni, Haplosporidium costale and Perkinsus marinus in the eastern oyster (Crassostrea virginica, Gmelin, 1971). Molecular and Cellular Probes 15: 385-390.
Perkins, F.O. 1976a. Dermocystidium marinum infection in oysters. Marine Fisheries Review 38(10): 19-21.
Perkins, F.O. 1976b. Zoospores of the oyster pathogen, Dermocystidium marinum. I. Fine structure of the conoid and other sporozoan-like organelles. The Journal of Parasitology 62: 959-974.
Perkins, F.O. 1988. Structure of protistan parasites found in bivalve molluscs. American Fisheries Society Special Publication 18: 93-111.
Perkins, F.O. 1969. Ultrastructure of vegetative stages in Labyrinthomyxa marina (=Dermocystidium marinum), a commercially significant oyster pathogen. Journal of Invertebrate Pathology 13: 199-222.
Perkins, F.O. 1996. The structure of Perkinsus marinus (Mackin, Owen and Collier, 1950) Levine, 1978 with comments on taxonomy and phylogeny of Perkinsus spp. Journal of Shellfish Research 15: 67-87.
Powell, E.N., J.M. Klinck and E.E. Hofmann. 1996. Modeling diseased oyster populations. II Triggering mechanisms for Perkinsus marinus epizootics. Journal of Shellfish Research 15: 141-165.
Power, A., B. McCrickard, M. Mitchell, E. Covington, M. Sweeney-Reeves, K. Payne and R. Walker. 2006. Perkinsus marinus in coastal Georgia, USA, following a prolonged drought. Diseases of Aquatic Organisms 73: 151-158.
Powell, E.N., K.A. Ashton-Alcox, J.N. Kraeuter, S.E. Ford and D. Bushek. 2008. Long-term trends in oyster population dynamics in Delaware Bay: regime shifts and response to disease Journal of Shellfish Research 27: 729-755.
Powell, E.N., J.M. Klinck, X. Guo, S.E. Ford and D. Bushek. 2011. The potential for oysters, Crassostrea virginica, to develop resistance to Dermo disease in the field: evaluation using a gene-based population dynamics model. Journal of Shellfish Research 30: 685-712.
Pydeski, J.R. and D. Bushek. 2008. The role of transmission and infection in establishing refugia from two protozoan oyster diseases in Delaware Bay. Journal of Shellfish Research 27: 1044. (Abstract).
Queiroga, F.R., L.F. Marques-Santos, H. Hégaret, P. Soudant, N.D. Farias, A.D. Schlindwein and P.M. da Silva. 2013. Immunological responses of the mangrove oysters Crassostrea gasar naturally infected by Perkinsus sp. in the Mamanguape Estuary, Paraíba state (Northeastern, Brazil). Fish and Shellfish Immunology 35: 319-327.
Ragone, L.M. and E.M. Burreson. 1993. Effect of salinity on infection progression and pathogenicity of Perkinsus marinus in the eastern oyster, Crassostrea virginica (Gmelin). Journal of Shellfish Research 12: 1-7.
Ragone Calvo, L.M. and E.M. Burreson. 1994. Characterization of overwintering infections of Perkinsus marinus (Apicomplexa) in Chesapeake Bay oysters. Journal of Shellfish Research 13: 123-130.
Ragone Calvo, L.M. and E.M. Burreson. 2000. Development and verification of a simple model for Perkinsus marinus abundance in Chesapeake Bay oysters. Journal of Shellfish Research 19: 661. (Abstract).
Ragone Calvo, L.M., R.L. Wetzel and E.M. Burreson. 2001. Development and verification of a model for the population dynamics of the protistan parasite, Perkinsus marinus, within its host, the eastern oyster, Crassostrea virginica, in Chesapeake Bay. Journal of Shellfish Research 20: 231-241.
Ragone Calvo, L.M., G.W. Calvo and E.M. Burreson. 2003a. Dual disease resistance in a selectively bred eastern oyster, Crassostrea virginica, strain tested in Chesapeake Bay. Aquaculture 220: 69-87.
Ragone Calvo, L.M., C.F. Dungan, B.S. Roberson and E.M. Burreson. 2003b. Systematic evaluation of factors controlling Perkinsus marinus transmission dynamics in lower Chesapeake Bay. Diseases of Aquatic Organisms 56: 75-86.
Ralph, G., J.E. Ward, S.M. Winnicki, W. Carden, B. Allam and B. Holohan. 2008. Development of experimental procedures for determining the role of marine aggregates in the transmission of Perkinsus marinus in the eastern oyster (Crassostrea virginica). Journal of Shellfish Research 27: 1045. (Abstract).
Rawson, P., X. Guo and S. Lindell. 2008. Cross-breeding and field trials of disease resistant eastern oysters. Journal of Shellfish Research 27: 1045-1046. (Abstract).
Ray, S.M. 1966. A review of the culture method for detecting Dermocystidium marinum, with suggested modifications and precautions. Proceedings of the National Shellfisheries Association 54: 55-69.
Ray, S.M. 1996. Historical perspective on Perkinsus marinus disease of oysters in the Gulf of Mexico. Journal of Shellfish Research 15: 9-11.
Ray, S.M., T.M. Soniat and E.V. Kortright. 2001. Dermowatch: a web-based approach for managing Perkinsus marinus disease of oysters. Journal of Shellfish Research 20: 553-554. (Abstract).
Reece, K.S. and E.M. Burreson. 2004. The role of molecular biology in international regulation: shellfish health. Bulletin of the Aquaculture Association of Canada 104-2: 51-56.
Reece, K.S., J.E. Graves, D. Bushek and W. Belle. 1997a. Molecular markers for the oyster pathogen Perkinsus marinus and preliminary population genetic analysis. Journal of Shellfish Research 16: 274. (Abstract).
Reece, K.S., M.E. Siddall, E.M. Burreson and J.E. Graves. 1997b. Phylogenetic analysis of Perkinsus based on actin gene sequences. The Journal of Parasitology 83: 417-423.
Reece, K.S., D. Bushek and J.E. Graves. 1997c. Molecular markers for population genetic analysis of Perkinsus marinus. Molecular Marine Biology and Biotechnology 6: 197-206.
Reece, K.S., D. Bushek and K.L. Hudson. 1999. Analysis of the geographic distribution of Perkinsus marinus strains. Journal of Shellfish Research 18: 320. (Abstract).
Reece, K.S., G.D. Brown, K.L. Hudson and K. Apakupakul. 2001a. Inter- and intra-specific genetic variation among Perkinsus species: implications for species identification and development of molecular diagnostics. Journal of the Shellfish Research 20: 554. (Abstract).
Reece, K.S., D. Bushek, K.L. Hudson and J.E. Graves. 2001b. Geographic distribution of Perkinsus marinus genetic strains along the Atlantic and Gulf coasts of the USA. Marine Biology 139: 1047-1055.
Reece, K.S., C.F. Dungan and E.M. Burreson. 2008. Molecular epizootiology of Perkinsus marinus and P. chesapeaki infections among wild oysters and clams in Chesapeake Bay, USA. Diseases of Aquatic Organisms 82: 237–248.
Remacha-Triviño, A., D. Borsay-Horowitz, C. Dungan, X. Gual-Arnau, J. Gómez-Leon, L. Villamil and M. Gómez-Chiarri. 2008. Numerical quantification of Perkinsus marinus in the American oyster Crassostrea virginica (Gmelin, 1791) (Mollusca:Bivalvia) by modern stereology. The Journal of Parasitology 94: 125–136.
Roberts, S., R. Smolowitz and R. Karney. 2008. Characterizing disease resistance in native oysters that have experienced disease pressure. Journal of Shellfish Research 27: 1048. (Abstract).
Robledo, J.A.F., D.J. Gauthier, C.A. Coss, A.C. Wright and G.R. Vasta. 1998. Species-specificity and sensitivity of a PCR-based assay for Perkinsus marinusin the eastern oyster, Crassostrea virginica: a comparison with the fluid thioglycollate assay. The Journal of Parasitology 84: 1237-1244.
Robledo, J.A.F., A.C. Wright, A.G. Marsh and G.R. Vasta. 1999. Nucleotide sequence variability in the nontranscribed spacer of the rRNA locus in the oyster parasite Perkinsus marinus. The Journal of Parasitology 85: 650-656.
Robledo, J.A.F., P. Courville, M.F.M. Cellier and G.R. Vasta. 2004. Gene organization and expression of the divalent cation transporter Nramp in the protistan parasite Perkinsus marinus The Journal of Parasitology 90: 1004–1014.
Robledo, J.A.F., E. Caler, M. Matsuzaki, P.J. Keeling, D. Shanmugam, D.S. Roos and G.R. Vasta. 2011. The search for the missing link: a relic plastid in Perkinsus? International Journal for Parasitology 41: 1217-1229.
Romestand, B., J. Torreilles and P. Roch. 2001. Production of monoclonal antibodies against the Protozoa, Perkinsus marinus: estimation of parasite multiplication in vitro. Aquatic Living Resources 14: 351-357.
Romestand, B., F. Corbier and P. Roch. 2002. Protease inhibitors and haemagglutinins associated with resistance to the protozoan parasite, Perkinsus marinus, in the Pacific oyster, Crassostrea gigas. Parasitology 125: 323-329.
Rothschild, B.J., J.S. Ault, P. Goulletquer and M. Héral. 1994. Decline of the Chesapeake Bay oyster population: a century of habitat destruction and overfishing. Marine Ecology Progress Series 111: 29-39.
Russell, S., S. Penna and R. French. 2000. Comparative evaluation of the multiplex PCR with conventional detection methods for Haplosporidium nelsoni (MSX), Haplosporidium costale (SSO), and Perkinsus marinus (Dermo) in the eastern oyster, Crassostrea virginica. Journal of Shellfish Research 19: 580-581. (Abstract). This abstract was repeated verbatum in Journal of Shellfish Research 19: 648.
Russell, S., S. Frasca Jr, I. Sunila and R.A. French. 2004. Application of a multiplex PCR for the detection of protozoan pathogens of the eastern oyster Crassostrea virginica in field samples. Diseases of Aquatic Organisms 59: 85-91.
Sabry, R.C., R.D. Rosa, A.R.M. Magalhães, M.A. Barracco, T.C.V. Gesteira and P.M. da Silva. 2009. First report of Perkinsus sp. infecting mangrove oysters Crassostrea rhizophorae from the Brazilian coast. Diseases of Aquatic Organisms 88: 13-23.
Sabry, R.C., T.C.V. Gesteira, A.R.M. Magalhães, M.A. Barracco, C. Guertler, L.P. Ferreira, R.T. Vianna and P.M. da Silva. 2013. Parasitological survey of mangrove oyster, Crassostrea rhizophorae, in the Pacoti River Estuary, Ceará State, Brazil. Journal of Invertebrate Pathology 112: 24-32.
Saldarriaga, J.F., M.L. McEwan, N.M. Fast, F.J.R. Taylor and P.J. Keeling 2003. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. International Journal of Systematic and Evolutionary Microbiology 53: 355-365.
Saunders, G.L., E.N. Powell and D.H. Lewis. 1993. A determination of in vivo growth rates for Perkinsus marinus, a parasite of the eastern oyster Crassostrea virginica (Gmelin, 1791). Journal of Shellfish Research 12: 229-240.
Scarratt, D. 2000. Oyster diseases and the internet: multi-agency team developes "Dermowatch". Shellfish World 1(4): 19.
Schott, E.J. and G.R. Vasta. 2003. The PmSOD1 gene of the protistan parasite Perkinsus marinus complements the sod2 Δ mutant of Saccharomyces cerevisiae, and directs an iron superoxide dismutase to mitochondria. Molecular and Biochemical Parasitology 126: 81-92.
Schott, E.J., W.T. Pecher, F. Okafor and G.R. Vasta. 2003a. The protistan parasite Perkinsus marinus is resistant to selected reactive oxygen species. Experimental Parasitology 105: 232-240.
Schott, E.J., J.-A.F. Robledo, A.C. Wright, A.M. Silva and G.R. Vasta. 2003b. Gene organization and homology modeling of two iron superoxide dismutases of the early branching protist Perkinsus marinus. Gene 309: 1-9.
Schott, E.J., J.A. Fernández-Robledo, M.R. Alavi and G.R. Vasta. 2008. Susceptibility of Crassostrea ariakensis (Fujita 1913) to Bonamia and Perkinsus spp. infections: potential for disease transmission between oyster species. Journal of Shellfish Research 27: 541-549.
Shaheen, A.A. 1999. Long-term survival of Perkinsus marinus cells outside its host. Journal of Shellfish Research 18: 320. (Abstract).
Shridhar, S., K. Hassan, D.J. Sullivan, G.R. Vasta and J.A. Fernández Robledo. 2013. Quantitative assessment of the proliferation of the protozoan parasite Perkinsus marinus using a bioluminescence assay for ATP content. International Journal for Parasitology: Drugs and Drug Resistance 3: 85-92.
Siddall, M.E., K.S. Reece, J.E. Graves and E.M. Burreson. 1997. ‘Total evidence' refutes the inclusion of Perkinsus species in the phylum Apicomplexa. Parasitology 115: 165-176.
Sokolova, I.M., J.D. Oliver and L.J. Leamy. 2006. An AFLP approach to identify genetic markers associated with resistance to Vibrio vulnificus and Perkinsus marinus in eastern oysters. Journal of Shellfish Research 25: 95-100.
Soniat, T.M. 1996. Epizootiology of Perkinsus marinus disease of eastern oysters in the Gulf of Mexico. Journal of Shellfish Research 15: 35-43.
Soniat, T.M. and J.D. Gauthier. 1989. The prevalence and intensity of Perkinsus marinus from the mid northern Gulf of Mexico, with comments on the relationship of the oyster parasite to temperature and salinity. Tulane Studies in Zoology and Botany 27: 21-27.
Soniat, T.M. and M.L. Koenig. 1982. The effect of parasitism by Perkinsus marinus on the free amino acid composition of Crassostrea virginica mantle tissue. Journal of Shellfish Research 2: 25-28.
Soniat, T.M. and E.V. Kortright. 1998. Estimating time to critical levels of Perkinsus marinus in eastern oysters, Crassostrea virginica. Journal of Shellfish Research 17: 1071-1080.
Soniat, T.M., E.V. Kortweight and S.M. Ray. 2000. Dermowatch: a new tool for managing Perkinsus marinus diseases in eastern oysters, Crassostrea virginica. Journal of Shellfish Research 19: 666. (Abstract). Information on the DermoWatch program is also available on line at: https://www.oystersentinel.org/.
Soniat, T.M., J.M. Klinck, E.N. Powell and E.E. Hofmann. 2006. Understanding the success and failure of oyster populations: climatic cycles and Perkinsus marinus. Journal of Shellfish Research 25: 83-93.
Soniat, T.M., E.E. Hofmann, J.M. Klinck and E.N. Powell. 2009. Differential modulation of eastern oyster (Crassostrea virginica) disease parasites by El Niño-Southern Oscillation and the North Atlantic Oscillation. International Journal of Earth Science (Geol Rundsch) 98: 99-114.
Soniat, T.M., J.M. Klinck, E.N. Powell and E.E. Hofmann. 2012. Understanding the success and failure of oyster populations: periodicities of Perkinsus marinus and oyster recruitment, mortality and size. Journal of Shellfish Research 31: 635-646.
Soudant, P. and F.L.E. Chu. 2001. Lipid class and fatty acid composition of the protozoan parasite of oysters, Perkinsus marinus cultivated in two different media. Journal of Eukaryotic Microbiology 48: 309-319.
Stelter, K., N.M. El-Sayed and F. Seeber. 2007. The expression of a plant-type ferredoxin redox system provides molecular evidence for a plastid in the early dinoflagellate Perkinsus marinus. Protist 158: 119-130.
Stickler, S.M., E. Wagner, V.G. Encomio and J.F. LaPeyre. 2001. Natural dermo resistance and its role in the development of hatcheries for the Gulf of Mexico. Journal of Shellfish Research 20: 557. (Abstract).
Sunila, I., R.M. Hamilton and C.F. Dungan. 2001. Ultrastructural characteristics of the in vitro cell cycle of the protozoan pathogen of oysters, Perkinsus marinus. The Journal of Eukaryotic Microbiology 48: 348-361.
Tall, B.D., J.F. La Peyer, J.W. Bier, M.D. Miliotis, D.E. Hanes, M.H. Kothary, D.B. Shah and M. Faisal. 1999. Perkinsus marinus extracellular protease modulates survival of Vibrio vulnificus in eastern oyster (Crassostrea virginica) hemocytes. Applied and Environmental Microbiology 65: 4261-4263.
Tanguy, A., X. Guo and S.E. Ford. 2004. Discovery of genes expressed in response to Perkinsus marinus challenge in eastern (Crassostrea virginica) and Pacific (C. gigas) oysters. Gene 338: 121-131.
Tasumi, S. and G.R. Vasta. 2007. A galectin of unique domain organization from hemocytes of the eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. Journal of Immunology 179: 3086-3098.
Tarnowski, M. (editor). 2003. Maryland Oyster Population Status Report 2002 Fall Survey. Maryland Department of Natural Resources, Fisheries Service. pp. 32.
Tarnowski, M. (editor). 2005. Maryland Oyster Population Status Report 2003 and 2004 Fall Surveys. Maryland Department of Natural Resources, Fisheries Service. pp. 33.
Taveekijakarn, P., T. Somsiri, S. Puttinaowarat, S. Tundavanitj, S. Chinabut and G. Nash. 2008 (2005). Parasitic fauna of rock oyster (Saccostrea forskali) cultured in Thailand. In: Bondad-Reantaso, M. G., C. V. Mohan, M. Crumlish, R.P. Subasinghe (eds.) Diseases in Asian Aquaculture VI, Colombo, Sri Lanka, 25-28 October 2005, pp. 335-342 (for electronic publication see http://www.fhs-afs.net/publications.htm).
Thompson, P., B. Rosenthal and M.P. Hare. 2008. Microsatellite analysis of Perkinsus marinus genotypes from Florida and New Jersey indicates limited parasite migration between populations. Journal of Shellfish Research 27: 1057. (Abstract).
Thompson, P.C., B.M. Rosenthal and M.P. Hare. 2011. An evolutionary legacy of sex and clonal reproduction in the protistan oyster parasite Perkinsus marinus. Infection, Genetics and Evolution 11: 598-609.
Tirard, C.T., R.M. Grossfeld, A.K. Volety and F.-L.E. Chu. 1995. Heat shock proteins of the oyster parasite Perkinsus marinus. Diseases of Aquatic Organisms 22: 147-151.
Ulrich, P.N., J.W. Ewart and A.G. Marsh. 2007. Prevalence of Perkinsus marinus (Dermo), Haplosporidium nelsoni (MSX), and QPX in Bivalves of Delaware's Inland Bays and quantitative, high-throughput diagnosis of Dermo by QPCR. Journal of Eukaryotic Microbiology 54: 520-526.
Vasta, G.R., J.D. Gauthier and A.G. Marsh. 1995. Molecular and biochemical adaptations of the protozoan pathogen Perkinsus marinus for its host, Crassostrea virginica: current research and future perspectives (or "the best defence is a good offence"). Journal of the Marine Biotechnology 3: 35-41.
Vasta, G.R., A.G. Marsh, J.D. Gauthier, A.C. Wright, J.A. Robledo, H. Ahmed, T.J. Burkett, G.M. Ruiz and C.A. Coss. 1997. Molecular basis for the etiology of Perkinsus marinus disease and development of PCR-based diagnostic assays. Journal of Shellfish Research 16: 277. (Abstract).
Venegas-Calerón, M., F. Beaudoin, O. Sayanova and J.A. Napier. 2007. Co-transcribed genes for long chain polyunsaturated fatty acid biosynthesis in the protozoon Perkinsus marinus include a plant-like FAE1 3-ketoacyl coenzyme A synthase. Journal of Biological Chemistry 282: 2996-3003.
Villalba, A., K.S. Reece, M.C. Ordás, S.M. Casas and A. Figueras. 2004. Perkinsosis in molluscs: A review. Aquatic Living Resources 17: 411-432.
Villamil, L., J. Gómez-León and M. Gómez-Chiarri. 2007. Role of nitric oxide in the defenses of Crassostrea virginica to experimental infection with the protozoan parasite Perkinsus marinus. Developmental and Comparative Immunology 31: 968-977.
Volety, A.K. and F.-L.E. Chu. 1994. Comparison of infectivity and pathogenicity of meront (trophozoite) and prezoosporangiae stages of the oyster pathogen Perkinsus marinus in eastern oysters, Crassostrea virginica (Gmelin, 1791). Journal of Shellfish Research 13: 521-527.
Volety, A.K. and F.-L.E. Chu. 1995. Suppression of chemiluminescence of eastern oyster (Crassostrea virginica) hemocytes by the protozoan parasite Perkinsus marinus. Developmental and Comparative Immunology 19: 135-142.
Volety, A.K. and F.-L.E. Chu. 1997. Acid phosphatase activity in Perkinsus marinus, the protistan parasite of the American oyster, Crassostrea virginica. The Journal of Parasitology 83: 1093-1098.
Volety, A.K. and W.S. Fisher. 2000. In vitro killing of Perkinsus marinus by hemocytes of oysters Crassostrea virginica. Journal of Shellfish Research 19: 600. (Abstract).
Volety, A., F.O. Perkins, R. Mann and P.R. Hershberg. 2000. Progression of diseases caused by the oyster parasite, Perkinsus marinus and Haplosporidium nelsoni, in Crassostrea virginica on constructed intertidal reefs. Journal of Shellfish Research 19: 341-347.
Wang, Y. and X. Guo. 2008. Mapping disease-resistance genes in the eastern oyster (Crassostrea virginica). Journal of Shellfish Research 27: 1061-1062. (Abstract).
Wang, S., E. Peatman, H. Liu, D. Bushek, S.E. Ford, H. Kucuktas, J. Quilang, P. Li, R. Wallace, Y. Wang, X. Guo and Z. Liu. 2010. Microarray analysis of gene expression in eastern oyster (Crassostrea virginica) reveals a novel combination of antimicrobial and oxidative stress host responses after dermo (Perkinsus marinus) challenge. Fish and Shellfish Immunology 29: 921-929.
White, M.E., E.N. Powell, S.M. Ray and E.A. Wilson. 1987. Host-to-host transmission of Perkinsus marinus in oyster Crassostrea virginica) populations by the ectoparasitic snail Boonea impressa (Pyramidellidae). Journal of Shellfish Research 6: 1-5.
White, D.L., D. Bushek, D.E. Porter and D. Edwards. 1998. Geographic information systems (GIS) and kriging: analysis of the spatial and temporal distribution of the oyster pathogen Perkinsus marinus in a developed and an undeveloped estuary. Journal of Shellfish Research 17: 1473-1476.
Willson, L.L. and L.E. Burnett. 2000. Whole animal and gill tissue oxygen uptake in the eastern oyster, Crassostrea virginica: effects of hypoxia, hypercapnia, air exposure, and infection with the protozoan parasite Perkinsus marinus. Journal of Experimental Marine Biology and Ecology 246: 223-240.
Wilson, E.A., E.N. Powell, M.A. Craig, T.L. Wade and J.M. Brooks. 1990. The distribution of Perkinsus marinus in Gulf Coast oysters: Its relationship with temperature, reproduction, and pollutant body burden. Internationale Revue der Gesamten Hydrobiologie 75: 533-550.
Wilson, E.A., E.N. Powell, T.L. Wade, R.J. Taylor, B.J. Presley and J.M. Brooks. 1992. Spatial and temporal distributions of contaminant body burden and disease in Gulf of Mexico oyster populations: the role of local and large-scale climatic controls. Helgoländer Meeresuntersuchungen 46: 201-235.
Winnicki, S.M., W. Carden, B. Holohan, G. Ralph, E. Ward and B. Allam. 2008. Establishment of Perkinsus marinus infection in Crassostrea virginica: insights into the potrtal of entry and the potential role of marine aggregates. Journal of Shellfish Research 27: 1064. (Abstract).
Winstead, J.T. and J.A. Couch. 1988. Enhancement of protozoan pathogen Perkinsus marinus infections in American oysters Crassostrea virginica exposed to the chemical carcinogen n-nitrosodiethylamine (DENA). Diseases of Aquatic Organisms 5: 205-213.
Wright, A.C., H. Ahmed, D.J. Gauthier, A.M. Silva and G.R. Vasta. 2002. cDNA cloning and characterization of two iron superoxide dismutases from the oyster parasite Perkinsus marinus. Molecular and Biochemical Parasitology 123: 73-77.
Xue, Q.-G., G.L. Waldrop, K.L. Schey, N. Itoh, M. Ogawa, R.K. Cooper, J.N. Losso and J.F. La Peyre. 2006. A novel slow-tight binding serine protease inhibitor from eastern oyster (Crassostrea virginica) plasma inhibits perkinsin, the major extracellular protease of the oyster protozoan parasite Perkinsus marinus. Comparative Biochemistry and Physiology Part B 145: 16–26.
Yarnall, H.A., K.S. Reece, N.A. Stokes and E.M. Burreson. 2000. A quantitative competitive polymerase chain reaction assay for the oyster pathogen Perkinsus marinus. The Journal of Parasitology 86: 827-837.
Yee, A., C. Dungan, R. Hamilton, M. Goedken, S. de Guise and I. Sunila. 2005. Apoptosis of the protozoan oyster pathogen Perkinsus marinus in vivo and in vitro in the Chesapeake Bay and Long Island Sound. Journal of Shellfish Research 24: 1035-1042.
Yu, Z. and X. Guo. 2006. Identification and mapping of disease-resistance QTLs in the eastern oyster, Crassostrea virginica Gmelin. Aquaculture 254: 160-170.
Yu, H., Y. He, X. Wang, Q. Zhang, Z. Bao and X. Guo. 2011. Polymorphism in a serine protease inhibitor gene and its association with disease resistance in the eastern oyster (Crassostrea virginica Gmelin). Fish and Shellfish Immunology 30: 757-762.
Zhang, H., D.A. Campbell, N.R. Sturm, C.F. Dungan and S. Lin. 2011. Spliced leader RNAs, mitochondrial gene frameshifts and multi-protein phylogeny expand support for the genus perkinsus as a unique group of alveolates. PLoS ONE 6(5): e19933. doi:19910.11371/journal.pone.0019933 (for electronic version see http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0019933).
Zhang, H., C.F. Dungan and S. Lin. 2011. Introns, alternative splicing, spliced leader trans-splicing and differential expression of pcna and cyclin in Perkinsus marinus. Protist 162: 154-167.
Citation Information
Bower, S.M. (2013): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Perkinsus marinus ("Dermo" Disease) of Oysters.
Date last revised: September 2013
Comments to Susan Bower
- Date modified: