Rickettsia of Crayfish
On this page
Category
Category 4 (Host Not in Canada)
Common, generally accepted names of the organism or disease agent
Rickettsia-like infection of crayfish.
Scientific name or taxonomic affiliation
- Rickettsia-like organism probably Coxiella cheraxi as described by Tan and Owens (2000).
- Rickettsia-like organism probably a second species given the tissue tropism as described below. ickettsia-like organisms - probably at least 2 species given the tissue tropism described below.
Geographic distribution
Freshwater streams in northern Australia and production farms in Queensland, Australia and Ecuador.
Host species
Cherax quadricarinatus.
Impact on the host
- Systemic infection contributed to the high mortalities that occurred in grow-out ponds on commercial farms in Australia (Ketterer et al. 1992, Tan and Owens 2000) and Ecuador (Jiménez and Romero 1997, Romero et al. 2000).
- The infection in one moribund C. quadricarinatus, from a farm experiencing chronic crayfish mortalities in north Queensland, Australia, appeared to be confined to the hepatopancreatic tubule epithelium (Edgerton and Prior 1999).
Diagnostic techniques
Gross Observations
Gross signs of infection were only reported for crayfish with systemic infections. Affected crayfish appear greenish-blue and are generally lethargic.
Histology
a) Prominent hyperplasia and hypertrophy of endothelial and interstitial cells in organs throughout the body, especially the gills. Infected cells include haemocytes in circulation and fixed phagocytes on the external side of the terminal hepatic haemolymph vessels. The cytoplasm of many of the hypertrophied cells contains large granular cytoplasmic inclusion bodies, which are strongly basophilic (haematoxylin and eosin stain). The basophilic cells are Gram-negative, Macchiavello-positive and negative for Ziehl-Neelsen stain. No infections were observed in the epithelial cells of the hepatopancreatic tubules.
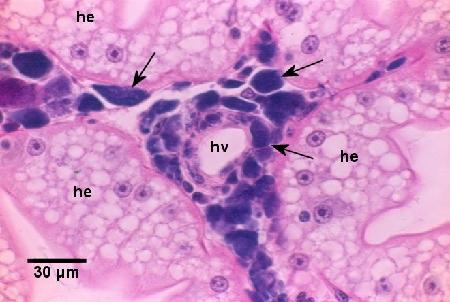
Figure 1. Infected haemocytes and fixed phagocytes containing basophilic colonies of Coxiella cheraxi (arrows) around a haemal vessel (hv) and surrounded by uninfected epithelial cells of the hepatopancrease (he). Note that there is no reaction (i.e., melanotic response) to the infection. Haematoxylin and eosin stain.
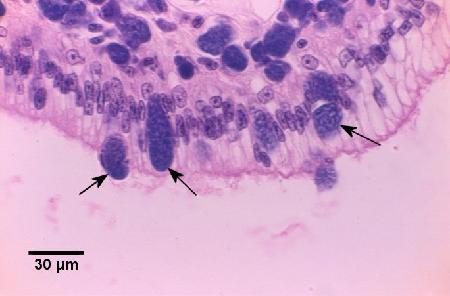
Figure 2. Diapedesis and release of basophilic colonies of Coxiella cheraxi (arrows) through the columnar epithelium of the foregut. Note that the underlying connective tissue contains many infected cells. Haematoxylin and eosin stain.
b) Basophilic inclusions were observed in the cytoplasm of hepatopancreatocytes of the tubules. The inclusions were Gram-negative, moderately PAS positive, intensely phloxophilic, and often had a clear central zone containing eosinophilic material. No infection was observed in the other tissues.
Electron Microscopy
a) Inclusion bodies consist of dense aggregates of Rickettsia-like organisms (rod-shaped) within a membrane-bound vacuole. Different developmental stages include a rod-shaped and electron dense elementary body (0.48 to 0.6 µm long and 0.3 µm in diameter) and an intermediate body (0.75 to 1.1 µm long and 0.36 to 0.44 µm in diameter). Some of the electron dense elementary bodies appeared to be free in the cytoplasm of infected cells.
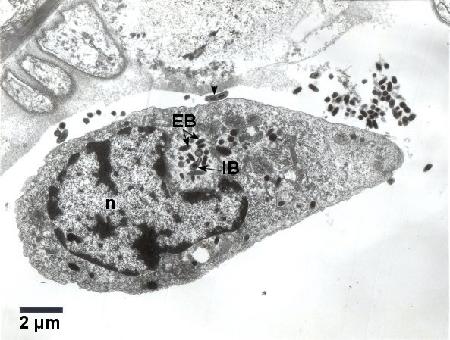
Figure 3. Colony consisting of electron dense elementary bodies (EB) and intermediate bodies (IB) of Coxiella cheraxi adjacent to the nucleus (n) of a haemocyte. An elongated IB with a central constriction (arrowhead) is located free in the haemolymph next to the infected haemocyte. Cold Karnovisky fixative; Uranyl acetate and lead citrate stain.
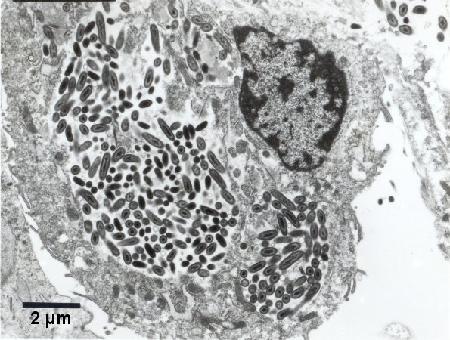
Figure 4. At least two colonies of Coxiella cheraxi within the cytoplasm of a haemocyte. Cold Karnovisky fixative; Uranyl acetate and lead citrate stain.
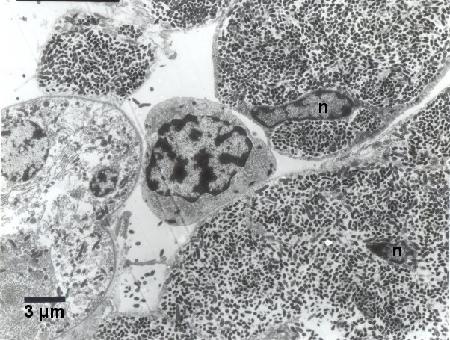
Figure 5. Hypertrophied haemocytes with distorted nuclei (n) heavily infected with Coxiella cheraxi adjacent to a relatively normal haemocyte (middle of image). Cold Karnovisky fixative; Uranyl acetate and lead citrate stain.
b) The cytoplasmic inclusions contsisted of rickettsia-like microcolonies within membrane-bound vacuoles. The rickettsia-like organisms (0.2 to 0.4 µm in length) had a cell wall and plasma membrane and were pleomorphic with round forms more common than rod-shaped forms (Edgerton and Prior 1999).
Methods of control
Poor water quality (including organic matter pollution) probably predisposes the crayfish to epibiont fouling and contributes to the severity of the disease. Vertical transmission, possibly via contact with contaminated faeces, is believed to be the normal route of transmission. Intramuscular injections of oxytetracycline apparently cured the broodstock of infection (Owens et al. 1992). Injecting the female broodstock seemed to prevent the vertical transmission of infection to the progeny while they remained attached to their mother for a few days after hatching. This treatment was applicable at a commercial scale (unpublished results, X. Romero, Subsecretaría de Acuacultura, Edif. Gobierno del Litoral Piso 12, Guayaquil, Ecuador, e-mail: xromero2001@yahoo.com).
References
Edgerton, B.F. and H.C. Prior. 1999. Description of a hepatopancreatic rickettsia-like organism in the redclaw crayfish Cherax quadricarinatus. Diseases of Aquatic Organisms 36: 77-80.
Edgerton, B.F., P. Henttonen, J. Jussila, A. Mannonen, P. Paasonen, T. Taugbíl, L. Edsman and C. Souty-Grosset. 2004. Understanding the cause of disease in European freshwater crayfish. Conservation Biology 18: 1466-1474.
Jiménez, R. and X. Romero. 1997. Infection by intracellular bacterium in red claw crayfish, Cherax quadricarinatus (Von Martens), in Ecuador. Aquaculture Research 28: 923-929.
Ketterer, P.J., D.J. Taylor and H.C. Prior. 1992. Systemic rickettsia-like infection in farmed freshwater crayfish, Cherax quadricarinatus. In: M. Shariff, R.P. Subasinghe and J.R. Arthur (eds.). Diseases in Asian Aquaculture. I. Fish Health Section, Asian Fisheries Society. Manila, Philippines, p. 173-179.
Owens, L., P. Muir, D. Sutton and M. Wingfield. 1992. The pathology of microbial diseases in tropical Australian Crustacea. In: Shariff, M., R.P. Subasinghe, J.R. Arthur (eds.) Diseases in Asian Aquaculture I. Fish Health Section, Asian Fisheries Society, Manila, Philippines. pp. 165-172.
Romero, X., J.F. Turnbull and R. Jiménez. 2000. Ultrastructure and cytopathology of a rickettsia-like organism causing systemic infection in the redclaw crayfish, Cherax quadricarinatus (Crustacea: Decapoda), in Ecuador. Journal of Invertebrate Pathology 76: 95-104.
Tan, K. and L. Owens. 2000. Infectivity, transmission and 16s rRNA sequencing of a rickettsia, Coxiella cheraxi sp. nov., from the freshwater crayfish Cherax quadricarinatus. Diseases of Aquatic Organisms 41: 115-122.
Citation Information
Bower, S.M., Romero, X. (2009): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Rickettsia of Crayfish.
Date last revised: February 11, 2009
Comments to Susan Bower
- Date modified: