Canadian Aquaculture R&D Review 2011
Table of Contents
Finfish – Salmon
Control of post-harvest myoliquefaction in farmed Atlantic Salmon
Soft-flesh syndrome presents a significant challenge to the fish–farming industry by compromising product quality and lending to a negative consumer stigma of farmed fish products. In farm–reared Atlantic Salmon (Salmo salar) the most common cause of soft-flesh is a parasitic infection by Kudoa thyrsites. At the moment, there are no available treatments to control K. thyrsites infection. Alternative technologies, such as high hydrostatic pressure (HHP) have been successful at controlling parasite infestation in other meat processing industries. In this project an industrial trial using HHP technology was tested as a means to control the manifestation of myoliquefaction caused by K. thyrsites infection. Whole fish and fillets were subjected to several pressure intensities, which were applied for different times. Myoliquefaction manifestation (presence and number of pits formation) was monitored daily for 5 consecutive days. Fish fillet quality parameters such as colour, texture, flesh integrity (gaping), and smell were evaluated in pressure treated and untreated fillet portions using standard operation procedures for quality control. The results demonstrated that the HHP technology was not effective at suppressing myoliquefaction, and it adversely affected product quality, including colour and texture.
July 2009 – Mar. 2010 • Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP), Marine Harvest Canada
Project team: Diane Morrison (Marine Harvest Canada), Luis Afonso (BC-Centre for Aquatic Health Sciences – BC-CAHS), Alexandra Eaves (BC-CAHS), Stewart Johnson (DFO – PBS), Tiffany MacWilliam (MHC)
Contact: Diane Morrison ( Diane.Morrison@marineharvest.com) Results of the Aquaculture Innovation and Market Access Program

Atlantic Salmon genome sequencing
Genome BC has partnered with the Chilean Economic Development Agency, "InnovaChile", the Norwegian Research Council, and the Norwegian Fishery and Aquaculture Industry Research Fund to sequence the Atlantic Salmon genome. Access to a well-annotated salmon genome will directly benefit the world's fisheries and aquaculture industries. The multi-phased project's goal is to produce a genome sequence that identifies and maps the genes in the Atlantic Salmon genome. This genome will then act as a reference and guide sequencing of the genomes of other salmonids, including Pacific salmon and Rainbow Trout, and more distantly related fish such as smelt and pike. Unlike the human and mouse genome sequencing projects, the Atlantic Salmon genome sequence will not be considered a "finished" sequence. The quality of the Atlantic Salmon sequence will be critical as it must be of sufficient quality to support detailed analyses, such as comparisons of duplicated regions within the genome and comparative genomics involving other fish species. The sequence will be used for the population management of wild fish stocks, food security and traceability, conservation of populations at risk and broodstock selection for commercially important traits.
Nov. 2009 – ongoing • Funded by: Genome British Columbia, The Research Council of Norway, The Norwegian Fishery and Aquaculture Industry Research Fund, The Chilean Economic Development Agency (CORFO), InnovaChile Committee
Contact:Mohammed Hasham ( mhasham@genomebc.ca) http://www.genomebc.ca/partners/international-collaborators/international-cooperation-to-sequence-the-atlantic-salmon-geno/
Discovery Passage plankton monitoring and juvenile salmon assessment
This collaborative research partnership is monitoring phytoplankton and zooplankton levels in the Northern Georgia Strait to determine optimal release dates for juvenile enhanced Coho Salmon with a goal of improving their survival. The project, which just completed its fourth year of data collection, is facilitated by the BC Centre for Aquatic Health Sciences (BC CAHS) in partnership with the Fisheries and Oceans Canada's Quinsam River Hatchery and the A-tlegay Fisheries Society.
Availability of food following hatchery release is crucial to the early survival of salmon. Through this project, BC CAHS and its partners are developing monitoring tools that will be used to establish the best time to release juvenile coho based on maximum productivity in the nearshore environment. The project's initial goal was to increase coho survival and subsequent returns to the Campbell and Quinsam Rivers (at the Quinsam Hatchery survival of enhanced coho had dropped from highs of 8-10% in the 1980s to less than 1%). The knowledge gained from this project can be applied to other enhancement hatchery facilities and river systems and can be adapted to the specific needs of other Pacific salmonids.
Annual reports for previous project years are available on the BC CAHS website.
Jan. 2010 – Dec. 2010 • Funded by: Campbell River Salmon Foundation, Aboriginal Fisheries Society, Positive Aquaculture Awareness, Marine Harvest Canada, City of Campbell River, the Campbell River and District Fishing Guides Association
Project team: Sonja Saksida (BC CAHS), Elan Downey (BC CAHS), Alexandra Eaves (BC CAHS), Shannon Anderson (DFO), Dave Ewart (Quinsam River Hatchery), Kim Duncan (A-tlegay Fisheries Society)
Contact: Sonja Saksida ( sonja.saksida@cahs-bc.ca) • http://www.cahs-bc.ca

Flesh quality of farmed and wild salmon from British Columbia
In January of 2004, a highly publicized study indicated that farmed Atlantic Salmon collected from supermarkets around the globe contained higher levels of lipophilic organohalogen contaminants (e.g., polychlorinated biphenyls (PCBs), and polychlorinated dibenzodioxins and furans (PCDD/Fs)) than wild salmon. Although the levels of contaminants in farmed Atlantic Salmon flesh were not a human health concern according to various national food safety regulatory guidelines, importation of farmed Atlantic Salmon to the United States decreased by 20% in the first quarter of 2004 due to public concerns over the adverse effects of these contaminants. Globally, demand for farmed products decreased despite the fact that many studies had shown positive effects of frequent fish consumption on cognitive development and reduced risk for some types of cancer, inflammatory diseases and coronary heart disease. Health benefits are enhanced by the consumption of oily fish such as salmon that are high in omega-3 highly unsaturated fatty acids (n-3 HUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). However, positive health benefits of an oily fish diet may be compromised by negative effects associated with the occurrence of contaminant residues such as PCBs, PCDD/Fs, pesticides, flame retardants, as well as mercury (Hg), lead (Pb), cadmium (Cd), and other trace metals.
This study investigated the presence of environmental contaminants (PCBs, PCDD/Fs, pesticides, PBDEs, mercury, and all trace elements) in the flesh of three species of farmed salmon and five species of wild salmon from coastal British Columbia (BC). The study provides information regarding current contaminant levels in the flesh of farmed and wild BC salmon and also examined the contaminants associated risks to benefits associated with the human consumption of salmon containing high amounts of DHA and EPA. Our findings indicate that current flesh levels of major contaminants that include trace metals such as Hg, Arsenic (As) and Cd and organohalogens (PCBs, PCDD/Fs) in farmed and wild salmon from BC are below the recommended levels of concern for human health. Thus, weekly consumption of BC salmon products that originate from farmed and wild sources is an excellent and safe way to obtain adequate dietary intake of n-3 HUFAs for cardio-protective and other human health benefits.
2003 - 2011 • Funded by: DFO – Aquaculture Collaborative Research and Development Program (ACRDP), AquaNet, BC Salmon Farmers Association, BC Science Council, and the former Federal Office of the Commissioner for Aquaculture Development
Project team:Michael G. Ikonomou (DFO), David A. Higgs (DFO – CAER), Keng Pee Ang (Cooke Aquaculture Inc.), Shannon Balfry (DFO – UBC), Cory Dubetz (DFO)
Contact:Michael G. Ikonomou ( Michael.Ikonomou@mpo-dfo.gc.ca) /aquaculture/acrdp-pcrda/index-eng.htm

New feeds dramatically reduce POPs in farmed salmon flesh
The high lipid content in farmed salmon diets (≤ 40%), traditionally provided primarily by marine fish oil (MFO), enhances the deposition of lipids and lipophilic persistent organic pollutants (POPs), found in MFO, relative to levels that are found in wild Pacific salmon. Nevertheless, the levels of POPs (e.g., polychlorinated biphenyl compounds (PCBs) and polychlorinated dibenzodioxins and furans (PCDD/Fs) in farmed salmon flesh are not a human health concern according to the US Food and Drug Administration and Health Canada guidelines. In recent years, several studies have been published which outline concerns associated with the consumption of fish containing high levels of contaminants, POPs, and heavy metals in particular. Yet there are a limited number of studies which link the contaminant-associated risks to the benefits related to the consumption of salmon, an excellent source of omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
The potential for using new diet formulations based on terrestrial lipids (TL), derived from a variety of plants (e.g., oilseeds such as soybeans and canola), and animal products (poultry fat), was investigated as a major replacement for MFOs in commercial salmon feeds. These alternate diets contained up to 65% lower ∑PCB concentrations compared to traditional diets containing MFOs. Utilization of these diets resulted in dramatic reductions in the concentrations of POPs in the flesh of the farmed salmon. For example, farm-raised Atlantic and Chinook Salmon fed the alternative lipid-based diets (i.e., 50% poultry fat or 35% canola oil), exhibited PCB and PCDD/F flesh concentrations that were 48 to 60% lower than those found for fish fed the traditional diets. Moreover, flesh TEQ (toxic equivalent concentrations with respect to 2,3,7,8-TCDD) levels in farm-raised salmon fed the alternative TL-based diets were found to be equivalent to or lower than those in wild Pacific salmon.
In the flesh of farmed Atlantic Salmon fed the alternative TL-based diets, levels of the omega-3 fatty acids DHA and EPA were up to 50% lower than those observed in farmed Atlantic Salmon fed the traditional diets but were still greater than those found in wild Pacific salmon. This study also showed that the recommended intake of 250 mg d-1 of EPA and DHA for humans can be achieved by eating two 100 g servings of farmed Atlantic Salmon each week. In contrast, this same daily intake of EPA and DHA would require either larger proportion sizes of wild Pacific salmon or more frequent servings from these sources each week.
2005 - 2011 • Funded by: DFO – Aquaculture Collaborative Research and Development Program (ACRDP), BC Salmon Farmers Association, BC Science Council, the former Federal Office of the Commissioner for Aquaculture Development
Project team:Michael G. Ikonomou (DFO – IOS), Erin N. Friesen (UBC) , David A. Higgs (DFO – CAER), Keng Pee Ang (Cooke Aquaculture Inc.), Cory Dubetz (DFO – IOS)
Contact:Michael G. Ikonomou ( Michael.Ikonomou@dfo-mpo.gc.ca) • /aquaculture/acrdp-pcrda/index-eng.htm

Early life stage salinity tolerance of wild and hatchery-reared juvenile Pink Salmon
Salinity tolerance in wild (Glendale) and hatchery (Quinsam) Pink Salmon Oncorhynchus gorbuscha (average mass 0.2 g) was assessed by measuring whole body sodium [Na+] and chloride [Cl-] concentration after 24 or 72 h exposures to freshwater (FW) and 33, 66 or 100% seawater (SW). Gill Na+, potassium [K+]-ATPase activity was measured following exposure to FW and 100% SW and increased significantly in both populations after a 24 h exposure to 100% SW. Whole body [Na+] and whole body [Cl-] increased significantly in both populations after 24 h in 33, 66 and 100% SW, where whole body [Cl-] differed significantly between Quinsam and Glendale populations. Extending the seawater exposure to 72 h resulted in no further increases in whole body [Na+] and whole body [Cl-] at any salinity, but there was more variability among the responses of the two populations. Percent whole body water (c. 81%) was maintained in all groups of fish regardless of salinity exposure or population, indicating that the increase in whole body ion levels may have been related to maintaining water balance as no mortality was observed in this study. Thus, both wild and hatchery juvenile O. gorbuscha tolerated abrupt salinity changes, which triggered an increase in gill Na+, K+- ATPase within 24 h. These results were also discussed in terms of the preparedness of emerging O. gorbuscha for the marine phase of their life cycle.
Feb. 2007 – Mar. 2011 • Funded by: British Columbia Pacific Salmon Forum, Natural Sciences and Engineering Research Council of Canada (NSERC)
Project team: A. M. Grant (UBC), M. Gardner (UBC), L. M. Hanson (UBC), A. P. Farrell (UBC), C. J. Brauner (UBC)
Contact: A. Grant ( amelia@zoology.ubc.ca) https://www.landfood.ubc.ca/anthony-farrell/
Growth and ionoregulatory ontogeny of wild and hatchery-raised juvenile Pink Salmon
Juvenile Pink Salmon (Oncorhynchus gorbuscha) enter seawater (SW) shortly following emergence. Little is known about growth and development during this life-history stage when sensitivity to sea louse exposure may be high, an issue that is of current concern in British Columbia. We tested the hypothesis that growth and ionoregulatory development were similar in hatchery-raised (Quinsam) and wild (Glendale and One's Point) juvenile Pink Salmon (measured over 22 weeks) followed by SW entry. Fish body mass increased from 0.20 ± 0.01 to 6.47 ± 0.37 g, with mean specific growth rates of 2.74% to 3.05% body mass•day-1 among the three groups. In all three groups, gill Na+-K+-ATPase (NKA) activity peaked at 12 μmol ADP•mg protein-1•h-1 following 8 weeks post-transfer to SW. Whole body Na+ and Cl- concentrations, which again did not differ among groups, were highest upon initial exposure to SW (approximately 70 mmol•kg wet mass-1) and declined over time as gill NKA activity increased, indicating that the hypo-osmoregulatory capacity was not fully developed following emergence and initial entry into SW. Thus, consistent with our hypothesis, few differences were observed between hatchery-raised and wild juvenile Pink Salmon reared under laboratory conditions. These baseline data may be important for future studies in determining the effects of sea lice on wild juvenile Pink Salmon.
Sept. 2009 – Apr. 2012 • Funded by: British Columbia Pacific Salmon Forum, Natural Sciences and Engineering Research Council of Canada (NSERC)
Project team: A. M. Grant (UBC), M. Gardner (UBC), L. Nendick (UBC), M. Sackville (UBC), A. P. Farrell (UBC), C. J. Brauner (UBC)
Contact: A. Grant ( amelia@zoology.ubc.ca) • https://www.landfood.ubc.ca/anthony-farrell/
Swimming performance and associated ionic disturbance of juvenile Pink Salmon determined using different acceleration profiles
Swimming performance was assessed in juvenile Pink Salmon, Oncorhynchus gorbuscha, (body mass <5.0 g) using five different protocols: four constant acceleration tests each with a different acceleration profile (rates of 0.005, 0.011, 0.021 and 0.053 cm s-2) and a repeated ramped-critical swimming speed test. Regardless of the swim protocol, the final swimming speeds did not differ significantly (P > 0.05) among swim tests and ranged from 4.54 to 5.20 body lengths s-1. This result supports the hypothesis that at an early life stage, O. gorbuscha display the same fatigue speeds independent of the swimming test utilized. Whole body and plasma [Na+] and [Cl-] measured at the conclusion of these tests were significantly elevated when compared with control values (P < 0.05) and appear to be predominantly associated with dehydration rather than net ion gain. Given this finding for a small salmonid, estimates of swim performance can be accurately measured with acceleration tests lasting <10 min, allowing a more rapid processing than is possible with a longer critical swim speed test.
Feb. 2007 – Mar. 2011 • Funded by: British Columbia Pacific Salmon Forum, Natural Sciences and Engineering Research Council of Canada (NSERC)
Project team: L. Nendick (UBC), A.Grant (UBC), M.Gardner (UBC), M.Sackville (UBC), A.P.Farrell (UBC), C.J.Brauner (UBC)
Contact: L.Nendick ( laura.nendick@gmail.com) • https://www.landfood.ubc.ca/anthony-farrell/
Genomic selection and association mapping of Atlantic Salmon populations
Aquaculture now produces all the Atlantic Salmon consumed by Canadians and a significant amount for export. However, disease in marine farmed stock is a major constraint affecting the sustainability and profitability of this industry. Disease has a direct impact on farm income through lost production and treatment costs and an indirect impact by influencing consumer demand. Fortunately the rate of genetic improvement for difficult traits such as disease resistance might be greatly improved by using a new method of animal breeding called genomic selection. We are using 5000 SNP genetic markers to determine which of the offspring produced by a disease-resistant family have inherited the disease–resistant alleles. We are testing whether genomic selection can improve growth rate in saltwater, rapid adaptation to seawater, disease resistance and delayed sexual maturity more rapidly than the conventional breeding program currently used at Cooke Aquaculture Inc. in New Brunswick. We will accomplish this within 3 years by using DNA samples and estimated breeding values archived over a four year period from past broodstock and their relatives, as well as the performance of their harvested siblings in seawater farm cages. We can then predict if greater genetic changes in these traits would accrue from genomic selection.
Oct. 2009 – Sept. 2012 • Funded by: NSERC Strategic Project Grant
Project team: Elizabeth Boulding (U of Guelph), Larry R. Schaeffer (U of Guelph), Jane Tosh (U of Guelph), Patricia T. Schulte (UBC), Keng P. Ang (Cooke Aquaculture Inc.), Alli Burton (Cooke Aquaculture Inc.), Jake Elliott (Cooke Aquaculture Inc.), Brian Glebe (DFO - SABS), Steve Leadbeater (DFO - SABS), Patrick O'Reilly (DFO - BIO), Bill Wolters (USDA, Maine)
Contact: Elizabeth Boulding ( boulding@uoguelph.ca) http://www.uoguelph.ca/ib/people/faculty/boulding.shtml

Study investigates historical and social dimensions of salmon aquaculture science
Salmon aquaculture has been a focus of environmental research for over two decades. This project is applying the tools of environmental history and science and technology studies to understand how this research has developed, and the roles it has played in public discussions regarding the industry. Several more specific objectives are also being pursued.
First, an environmental history of salmon aquaculture science is being written. This history will explore the relations between scientific research and the evolving environmental, social, and political dimensions of the industry. Second, the project is examining how the diverse institutions engaged in environmental research – governments, universities, industry, and public interest organizations – have shaped research priorities, research results, and the application of these results. Third, the movement of scientific knowledge of salmon aquaculture between research sites in Canada, Norway, Ireland, and Scotland is being investigated. Fourth, the project is investigating the prospects for effective science that is able to contribute to resolution of controversies regarding this industry. While this project is examining the full range of environmental science relating to salmon aquaculture, a special focus is on research relating to sea lice.
Jun. 2007 – Jul. 2011 • Funded by: Social Sciences and Humanities Research Council of Canada, Genome BC
Project team: Stephen Bocking (Trent University)
Contact: Stephen Bocking ( sbocking@trentu.ca)
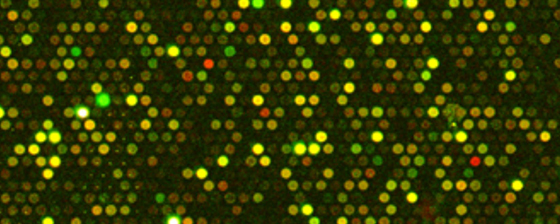
Determination of global gene expression patterns in Atlantic Salmon (Salmo salar) fed with novel fish feed ingredients
Seafood transformation and processing plants produce large amounts of commercially marketable products each year; however, an appreciable amount of by-products are discarded or transformed in fish meal. These marine by-products contain valuable amounts of proteins, lipid fractions, vitamins or other bioactive molecules having potential beneficial properties that could be used in human or animal nutrition.
As part of a larger study looking at the development and commercialization of marine by-products for use in animal and human nutrition and its application to the prevention of human diseases, this specific study aims to evaluate the potential health benefit and growth effects in Atlantic Salmon fed with novel fish feed formulations each supplemented with a specific marine by–product. For this purpose, DNA microarrays will be used to measure the global gene expression response and to look at the specific gene response in various targeted pathways such as oxidative stress, inflammation, immunity, lipid metabolism and others, in an attempt to identify the most promising marine byproducts. Results obtained will be correlated with digestibility data from another study being done in parallel and fish feed formulation showing the most interesting results will be further studied at the physiological level.
Jan. 2010 – Sept. 2011 • Funded by: Atlantic Canada Opportunity Agency – Atlantic Innovation Fund, Institut de recherche sur les zones côtières inc. (IRZC)
Project team: Sébastien Plante (IRZC), Francis LeBlanc (DFO), Mark Laflamme (DFO), Nellie Gagné (DFO), France Béland (IRZC), Sébastien Plante (UMCS), Jacques Gagnon (IRZC), Nadia Tchoukanova (IRZC)
Contact: Sébastien Plante ( sebastien.plante@umcs.ca)
Transcriptional response comparison of naturally immunized ISAV resistant Atlantic Salmon and naïve Atlantic Salmon challenged with a highly virulent ISAV isolate
Following an infection with a specific pathogen, the acquired immune system of many teleostean fish, including salmonids, is known to retain a specific memory of that agent, which protects the host against a subsequent infection. For example, Atlantic Salmon that survive an infection from a low-virulent infectious salmon anemia virus (ISAV) isolate are protected against a subsequent infection with a highly virulent ISAV isolate. An understanding of the mechanisms and immunity components involved in this acquired protection against ISAV is fundamental for the development of efficacious vaccines and treatments against this pathogen. Thus, in an attempt to better understand the immunity components involved in the observed resistance, we have used an Atlantic Salmon DNA microarray and qRT-PCR assays to study the global gene expression responses of naturally immunized Atlantic Salmon during the course of a new infection with a highly virulent ISAV isolate. Global gene expression patterns in immunized fish versus that of naïve fish, following infection by either cohabitation with infected fish or by direct intra-peritoneal injection of the virus will be studied. The results obtained from this study will greatly enrich our already acquired knowledge on ISAV-Atlantic Salmon interactions and could be useful in the creation of novel vaccines and treatments.
Apr. 2008 – Mar. 2011 • Funded by: DFO - Genomics Research and Development Initiative (GRDI)
Project team: Francis LeBlanc (DFO), Jean-René Arseneau (DFO), Brian Glebe (DFO – SABS), Steven Leadbeater (DFO – SABS), Mark Laflamme (DFO), Nellie Gagné (DFO)
Contact: Nellie Gagné ( Nellie.Gagne@dfo-mpo.gc.ca)
Phototherapy: applications for growth enhancement and maturation delay in farmed Bay of Fundy Atlantic Salmon (Salmo salar)
Maturation (grilsing) rates in farmed salmon steadily increased from less than 1% in 1978 (average among the first salmon farms) to greater than 30% at some farming sites in 2001. This change resulted in significantly reduced farm gate sales and prompted the industry to give high priority to research into methods to decrease grilsing.
In 2001, the first Aquaculture Collaborative Research and Development Program (ACRDP) funded study on the effect of artificial photoperiod on grilsing rates was initiated at two commercial salmon farms. A group of cages had submerged lights turned on in November. A second set of cages had lights turned on in February. Lights were left on 24 hours per day. All lights were turned off in May. Additional cages, exposed to only natural photoperiod, served as controls. During the first month, specific growth rates decreased in the November-lit cages. However, by the end of May, November-lit cages showed significantly higher growth rates than the control cages. At the first farm, an average of only 1% of the salmon in the November-lit cages matured compared to 11% and 21% of the fish in the February-lit and -unlit control cages, respectively. On the second farm, 5% of the salmon from October-lit cages matured compared to 17.5% in the unlit control cages.
An economic benefit analysis of the improved growth and delayed maturation due to the use of an artificial photoperiod showed a saving of up to $100,000 per cage (based on the November photoperiod adjustment and assuming a cost of lighting equipment purchase and operation of $5,000 per cage).
A recent follow-up of an ACRDP study involving four farms, confirmed the effectiveness of light treatment for reducing grilsing in salmon and a more cost-effective artificial lighting regime was developed. Also, an ELISA biomarker, developed for this project, was shown to be valuable for assessing photoperiod regime effectiveness during critical periods of maturation onset in salmon.
Apr. 2009 – Mar. 2011 • Funded by: DFO – Aquaculture Collaborative Research and Development Program (ACRDP), Jail Island Salmon, Cooke Aquaculture Inc.
Project team: Brian Glebe (DFO – SABS), Tony Manning (Research and Productivity Council), Keng P. Ang (Cooke Aquaculture Inc.)
Contact: Brian Glebe ( Brian.Glebe@dfo-mpo.gc.ca) /aquaculture/rp-pr/acrdp-pcrda/index-eng.html
- Date modified: