Canadian Aquaculture R&D Review 2013
Table of Contents
Miscellaneous
Isolation, culture, and genomic analysis of harmful algal species affecting aquaculture on the west coast of Canada and analysis of the Harmful Algae Monitoring Program historical database
HABs (Harmful Algal Blooms) are responsible for considerable economic losses due to cultured finfish/shellfish mortalities and toxic HABs in shellfish can threaten human health. With the support of the British Columbia (BC) salmon aquaculture industry, the Harmful Algae Monitoring Program (HAMP) was established in 1999 to address the devastating effect of harmful algae on farmed fish. Through systematic microscopic surveillance of water samples, HAMP has provided salmon aquaculture companies with monitoring data and early warning of HABs at farm sites. However, more research is needed to improve the knowledge of HABs as well as the ability to predict future blooms based on analysis of historical data. Researchers need to identify certain HAB species, cultivate these species for study, and analyze previous HAMP data on harmful algae to document potential trends. The first goal of this project is to isolate and culture microalgal species that are known to be harmful to cultured fish and shellfish in BC. These cultures will be used for detailed identification of species, preliminary studies of physical variations for each select species, development of species-specific quantitative polymerase chain reaction (PCR) assays, and creation of an image gallery and database of local harmful algae. These tools will be used to improve existing training and surveillance strategies. The second goal of this project is to carry out genomic analyses on shellfish gill tissues to determine genomic and biological responses to HABs and to link these responses with particular algal species. Finally, a retrospective analysis of 13 years of data from HAMP will be undertaken. This will significantly improve knowledge on spatial and temporal trends of local harmful alga blooms and will contribute to improving existing HAB surveillance and mitigation strategies.
July 2012 – July 2014
Funded by: DFO – Aquaculture Collaborative Research and Development Program (ACRDP) co-funded by: Creative Salmon Company Ltd.; Grieg Seafood BC Ltd.; Mainstream Canada; Marine Harvest Canada Inc.; Cleanwater Shellfish Ltd.; Island Scallops Ltd.; Little Wing Oysters Ltd.; Mac’s Oysters Ltd.; Nelson Island Sea Farms Ltd.; Taylor Shellfish Canada ULC
project lead: Chris Pearce (DFO – PBS)
project team: Svetlana Esenkulova, Nicky Haigh (Microthalassia Consultants Inc.); Laurie Keddy, Erin McClelland, Kristi Miller, Amy Tabata (DFO – PBS); Barb Cannon (Creative Salmon Company Ltd.); Tim Lelliott (Grieg Seafood BC Ltd.); Peter McKenzie (Mainstream Canada); Gordy McLellan (Mac’s Oysters Ltd.); Alex Munro (Taylor Shellfish Canada ULC); Yves Perreault (Little Wing Oysters Ltd.); Rob Saunders (Island Scallops Ltd.); Glenda and Henry Syrjala (Cleanwater Shellfish Ltd.); Dean Trethewey (Marine Harvest Canada Inc.); Bill Vernon (Nelson Island Sea Farms Ltd.)
Contact: Chris.Pearce@dfo-mpo.gc.ca

Aboriginal principles for sustainable aquaculture program phase 3: branding and marketing
The Aboriginal Aquaculture Association (AAA) has developed the Aboriginal Principles for Sustainable Aquaculture (APSA) to further develop an integrated, aboriginal management and certification program for aquaculture in Canada.
Phase 2 of the project focuses on the audit processes required to lend credibility to the APSA. It includes a defendable audit approach and the use of qualified, professional auditors to determine the level of conformance to the Association’s Standard. Currently work continues on Phase 2 of the project.
This project, Phase 3 will expand the reach of APSA and move the program closer to the market place by establishing a brand and a marketing strategy which would include promotion of the program to First Nations and industry. The inclusion of First Nation values into a management framework has not yet been fully realized and as a consequence has resulted in much of the developmental constraints faced by industry in Canadian coastal regions, particularly BC.
This project will further engage First Nations, industry and retailers with the result that all aquaculture industry sectors will have the ability to participate and demonstrate sustainability with respect to First Nation values, expectations and interests.
June 2011 – Dec. 2012
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP)
project lead: Chief Richard Harry (AAA)
collaborators: Mainstream Canada
Contact: richard@aboriginalaquaculture.com

Integrating red macroalgae into land-based marine finfish aquaculture
Land-based ‘closed’ marine culture systems seem to some to be an attractive alternative to open sea-cage systems, preventing escapees and allowing proper management of solid waste and pathogens. However, the eutrophication risk from dissolved nitrogen (N) and phosphorus in the effluent remains unless plants are integrated into the production cycle. We quantified N uptake by Irish Moss (Chondrus crispus) and Dulse (Palmaria palmata) between 1 – 18 °C in tumble culture in lab- and also pilot-scale trials at Scotian Halibut Ltd. Experimental factors included stocking density, light intensity, nutrient concentration and intermittent aeration. We estimate bioremediation of 50% of the N excreted by 100 tonnes of farmed halibut in winter and summer could be achieved by growing 100 and 600 tonnes of macroalgae, respectively.
Electricity/compressed air costs and rearing space requirements for tumble culture indicate the integration of red macro-algae on land-based farms could only be commercially viable if value could be added to the fresh Dulse or Irish Moss.
Mar. 2009 – Nov. 2012
Funded by: NSERC Strategic Grant
project lead: Jim Duston
project team: D. Garbary (StFX); P. Corey (Scotian Halibut Ltd.); J. Manriquez, S. Caines, J. Kim, B. Prithiviraj (Dalhousie U.)
collaborators: Scotian Halibut Ltd.
Contact: jduston@dal.ca



Brown Alga culture trials on the Gaspé Peninsula and the Magdalen Islands, Québec: pre-industrial scale-up in open-water and semi-enclosed environments of mussel farms
With the work done since 2006 in collaboration with Les Gaspésiennes inc., a number of steps towards mastering brown algae culture techniques have been completed. These include artificial induction of sporogenesis, gametophyte culture in vitro, routine production of 2 mm plants in four weeks, the ability to maintain cultures below the surface to protect against drifting ice and unfavourable surface layer conditions in the summer, and at-sea transfer of 2 mm plants in late autumn to obtain yields of 2 to 4.5 kg wet weight by meter of line in early July. These results were obtained from small-scale trials in Paspébiac, Québec, but as yet, it is not known if they can be reproduced on a larger scale or in other culture environments; recently, interest in kelp culture was expressed by Magdalen Islands mussel producers. The work that was done in the two last years on Saccharina longicruris culture was designed to answer the following questions: 1) are culture yields measured in 2008 – 2009 reproducible; 2) are small-scale yields (one 50 m longline) reproducible on a pre-industrial scale; 3) are yields obtained in a small semi-exposed culture site in an open environment reproducible in the Magdalen Islands lagoons; 4) when is the best time to transfer plants at sea; 5) is there a threat that algae grown in the Magdalen Islands lagoons be colonized by the bryozoa Membranipora membranacea; 6) what changes have to be made to a mussel farm to incorporate kelp culture; and 7) for larger scale operations, what are the production costs for hatchery plants and how much time is associated to the work at-sea?
Apr. 2010 – Mar. 2012
Funded by: DFO – Aquaculture Research and Development Program (ACRDP) co-funded by: SODIM; Ministère du Développement Économique, Innovation et Exportation (MDEIE)
project lead: Louise Gendron (DFO)
project team: Éric Tamigneaux (UQAR); Bruno Myrand (Centre maricole des Iles-de-la-Madeleine (CeMIM))
collaborators: Les Gaspésiennes inc., Les Moules de culture des Îles inc.
Contact: Louise.Gendron@dfo-mpo.gc.ca
Assessment of the European Green Crab as a fishmeal replacement for salmonid aquaculture diets
This project will provide the preliminary data to adapt a fisheries approach to mitigate two growing concerns: 1) the management of a non-native invasive species; and 2) the increasingly costly exploitation of wild-caught fish for commercial diets used in aquaculture industries.
Specifically, this project will provide catch data that will be used to develop a fisheries plan to control a nuisance species and produce a feed ingredient that can partially substitute wild-caught fishmeal in commercial salmon diets for the maritime region. The European Green Crab, an invasive alien species that has been negatively affecting several commercial fisheries and ecosystems in PEI, is high in protein and carotenoids making it a good candidate replacement for fishmeal.
May 2012 – May 2013
Funded by: Innovation PEI
project lead: Sophie St-Hilaire (UPEI)
project team: Mary McNiven, Pablo Quijon, Jeff Davidson (UPEI)
collaborators: Fran Hansen (PEI Shellfish Association)
Contact: Ssthilaire@upei.ca
Development of health products derived from Atlantic Canada bio-resources — Nutritional analysis of feeds containing SDA-enriched oil for Atlantic Salmon aquaculture
Like all vertebrates, fish have a limited ability to synthesize linoleic (18:2n-6) and linolenic (18:3n-3) acid. These two fatty acids are important because they are precursors of C20 and C22 polyunsaturated fatty acids (PUFA). The desaturation and elongation activities required for C18 PUFA synthesis from shorter carbon chain precursors are minimal in these animals. As a result, these C18 fatty acids must come from the diet. Stearidonic acid (SDA, 18:4n-3) is an intermediary in the biosynthetic conversion of linolenic acid (LNA, 18:3n-3) into eicosapentanoic acid (EPA, 20:5n-3) which can lead to tissues enrichment with carbon-20 and carbon-22 (C20 and C22) fatty acids. We are currently testing a new oil (Ahiflower™ oil) that is naturally rich in stearidonic acid and that could be used as a partial or complete replacement for traditional fish oil. It is well-known that availability of fish oil is decreasing while its demand is increasing. Also, fish oil often contains organic contaminants. Ahiflower™ oil can therefore mimic the beneficial effects associated with n-3 PUFA in fish oil.
Jan. 2008 – Dec. 2013
Funded by: ACOA – AIF co-funded by: UMCM; CZRI; UMCS
project lead: Sébastien Plante (UMCS)
project team: Marc Surette, Martin Fillion (UMCM)
collaborators: France Béland (CZRI)
Contact: sebastien.plante@umoncton.ca

Early warning and mitigation of the impact of invasive colonial ascidian tunicates
We have recently discovered two non-indigenous ascidians, Botryllus schlosseri and Botrylloides violaceus, in several harbours on the south coast of Newfoundland. Since both of these species are pests of aquaculture operations elsewhere, we designed an interdisciplinary research program focused on developing gene tools for early detection and determination of ecological factors predicting invasion fitness and future spread. Our goal is to provide information to government agencies responsible for zonal closure and mitigation measures.
The seasonal life cycle of B. schlosseri is being determined in Arnold’s Cove, Newfoundland, including somatic growth and the rates and timing of asexual and sexual reproduction and larval recruitment. We found that growth, reproduction, and recruitment are constrained by the short growing season in subarctic Newfoundland waters. Somatic growth begins in June, sexual reproduction in late July, and recruitment occurs from August to October; all consistent with temperature-dependent predictions from the temperate native range of B. schlosseri. Thus, this species has not become genetically adapted to the short growing season in Newfoundland. However, overwintering survival of colonies is high, contributing to recruitment in the following summer.
Since mitigation is more effective when applied early in an invasion, TaqMan assays are being developed with sufficient sensitivity to detect a single egg or larva in a plankton sample. Assays have been successfully developed for B. violaceus and are currently being developed for B. schlosseri. Gene sequences will also be used to infer source populations of the Newfoundland invaders.
Apr. 2009 – Mar. 2013
Funded by: Natural Sciences and Engineering Research Council of Canada (NSERC) co-funded by: DFO
project lead: Don Deibel (MUN)
project team: Cynthia McKenzie (DFO); Matthew Rise, Ray Thompson (MUN)
collaborators: Newfoundland Aquaculture Industry Association; Newfoundland Department of Fisheries and Aquaculture; DFO
Contact: ddeibel@mun.ca
Canadian-Aquaculture-Styrofoam®-Encasement (CASE)
This demonstration project will encapsulate existing spray foam floats in a novel affordable sea-worthy plastic. It will extend the equipment life span and address aquaculture related pollution due to the breakdown of Styrofoam® in marine environments. The project addresses a long standing challenge for shellfish farming.
Styrofoam® has long been used by the aquaculture industry to provide lightweight inexpensive flotation. Unfortunately the sun and the brine have been breaking it down into microscopic inorganic particles dispersed by wind and tide.
By encapsulating existing Styrofoam® floats in a shell of rugged seaworthy plastic, refurbishing them via the CASE technique can extend the life of the floats for many more decades of use, plus end the breakdown of foam nodules into the water column and onto the beaches.
The exciting aspect of this project is the affordability for operations of all sizes. An AIMAP investment combined with an industry investment will result in the development and adoption of this innovative solution, increasing environmental performance of existing shellfish rafts and providing aquaculture waste control. This will make aquaculture more sustainable by protecting the health of Canada’s aquatic ecosystems.
Apr. 2012 – Mar. 2013
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: West Coast Spray Foam
project lead: Julia Rendall (Bee Islets Growers Corp.)
project team: John Shook, Bob Tracy, Roy Tippenhauer, Sandra Wood (Bee Islets Growers Corp.)
Contact: jumarcortes@msn.com
Identifying critical ecological thresholds for tunicate infestation on mussel farms
Bio-fouling is a well documented shellfish aquaculture industry challenge and the recent invasion of several invasive tunicate species has greatly inflated its impact. The PEI mussel aquaculture industry has been particularly affected by the invasion of four new tunicate species: Clubbed Tunicate (Styela clava) in 1998 in the eastern end of the province; Golden Star Tunicate (Botryllus schlosseri) in 2001 and 2002 and Violet Tunicate (Botrylloides violaceus) were both reported on the north coast of PEI; and in 2004, the Vase Tunicate (Ciona intestinalis) was first reported on the east coast of the island. Efforts to control the spread of these tunicate species have been relatively successful, but most mussel producing areas in PEI remain infested with at least one tunicate species. Currently, high pressure water spray is the main technique used by the mussel industry to control tunicate fouling. Lime dipping is also used to control tunicates, particularly the Clubbed Tunicate, on mussel socks. Efforts to develop the most cost-effective treatment strategy are continuing while specific ecological thresholds are not yet established. The establishment of both economical and ecological thresholds is central to the sustainability of the mussel industry in PEI and could lead to the creation of the first Integrated Pest Management approach for Aquatic Invasive Species. The goal of this project is to investigate the ecological impact of tunicate treatment with the following objectives: 1) develop a method to estimate the tunicate biomass on mussel farm structures; 2) develop a model to predict the impact of tunicate fall-off, pre- and post-treatment, on the benthic environment; and 3) assess the impact of tunicate filtration and biodeposition on ecosystem productivity.
In 2011, habitat surveys were conducted in St-Mary’s Bay, PEI to describe and evaluate the aquaculture producing and the critical habitat (eelgrass beds and shellfish reefs) areas. These consisted of remote sensing (LIDAR) surveys and direct field validations. Preliminary observations were also made during treatment operations to assess the area affected by the high pressure water treatment system. In addition, data from previous studies were gathered to develop and validate a population dynamic model for the Vase Tunicate.
During the winter of 2012, lab experiments were conducted to assess the impact of decomposing tunicate on the sediment. Field trials were conducted during the summer in St-Mary’s Bay to assess the impact of tunicate treatments on the sea floor.
July 2011 – Mar. 2013
Funded by: DFO – Program for Aquaculture Regulatory Research (PARR)
project lead: Thomas Landry (DFO)
project team: Andrea Locke, Chris McKindsey, Monique Niles, Daniel Bourque, Thomas Guyondet, Luc Comeau (DFO); Jeff Davidson, Thitiwan Patanastienkul (AVC – UPEI); Aaron Ramsay (PEI DFARD)
Contact: Thomas.Landry@dfo-mpo.gc.ca
Assessment of a new natural ingredient for growth and pigmentation in Atlantic Salmon flesh
Farming carnivorous fish depends largely on world fish meal and fish oil supplies. It is now well established that these key ingredients come from a heavily exploited fishery. Great strides have already been made in developing alternate protein and lipid sources.
The pink colour of salmonid flesh is also a key economic issue in fish farming. Currently, this colour is obtained by adding artificial pigments to feed formulations. Although these pigments are considered fit for human consumption, these products require the display of “artificial” or “added colour” label.
The objective of this project was to test the effects of a shrimp hydrolysate added to feed formulation on flesh growth and pigmentation in Atlantic Salmon. In addition to being an excellent source of natural astaxanthin (600 mg/kg), this innovative ingredient is also rich in protein (70%) and lipids (16%). Three feeds were tested: 1) a pigment-free control feed; 2) a shrimp hydrolysate-based feed; and 3) a control feed containing an added artificial astaxanthin source.
Apr. 2009 – Mar. 2014
Funded by: ACOA-AIF co-funded by: CZRI
project lead: Sébastien Plante (UMCS)
project team: Sébastien Plante (UMCS); Jacques Gagnon, Nadia Tchoukanova (CZRI)
collaborators: France Béland (CZRI); Mary McNiven (UPEI)
Contact: sebastien.plante@umoncton.ca

Aquaculture technology implementation
Newfoundland Aqua Services Ltd. (NAS) has already undertaken a pilot project to examine technology alternatives and to inform the establishment of a commercial land-based netwashing operation to serve the Coast of Bays finfish aquaculture industry in NL. The pilot project has facilitated an assessment of two of the key pieces of technology (a drum washer and filtration system).
Other major marine finfish aquaculture jurisdictions have moved their netwashing activities onshore to land-based sites to mitigate the biosecurity risk. NAS has recently undertaken a review of netwashing practices and protocols in Norway, New Brunswick and British Columbia, and now plans to implement a land-based netwashing system in the Coast of Bays at a location in Milltown, NL.
NAS decided to move toward purchasing a larger drum washer, 30 m³ capacity as well as a vacuum bag system that impregnates the nets with anti-foulant evenly and then removes excess anti-foulant from the nets through the vacuum bag system. The vacuum bag technology, recently developed in Norway, will be new to the aquaculture industry in North America.
Apr. 2011 – Dec. 2012
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: ACOA
project lead: Boyd Pack (Newfoundland Aqua Service Ltd.)
project team: Ann Strickland, Owen Cox (Newfoundland Aqua Service Ltd.)
Contact: boydpack@newfoundlandaqua.com
www.newfoundlandaqua.com

Net drying innovation in aquaculture servicing
This project is a component of a multi-year initiative to eliminate open ocean netwashing through the construction of a closed land-based facility. A structured approach has been employed by Newfoundland Aqua Service Ltd. (NAS) to evaluate and adapt the best technologies for each operational component of the commercial land-based facility from net washing, net disinfection, sold waste management, liquid waste management, and net drying.
After the cleaning and disinfection process, nets are treated with copper-based antifoulant and dried before they are ready for use. Previously, NAS relied upon natural air-drying by hanging the nets on large poles out of doors and use of electrical fans in a net drying building to ‘blow-dry’ nets. This arrangement, which is also utilized in some other jurisdictions, is weather dependent, slow, and not suitable for the requirements and timing of customers.
NAS has investigated drying technologies and has worked closely with Geo-Xergy Systems Inc. of Winnipeg. NAS intends to utilise geothermal sourced heat in the drying process. Geo-Xergy’s technology is a cost-effective and environmentally friendly process to dry the treated nets.
The initiative is a key component of biosecurity plans for the industry and a strategic piece of private sector infrastructure required for the salmonid industry.
Apr. 2012 – Mar. 2013
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP)
project lead: Boyd Pack (Newfoundland Aqua Service Ltd.)
project team: Boyd Pack, Ann Strickland, Owen Cox (Newfoundland Aqua Service)
collaborators: Geo-Xergy Systems Inc.
Contact: boydpack@newfoundlandaqua.com
www.newfoundlandaqua.com
Research & development coordinator training in environmental management systems to assist aquaculture industry certification
The level of document systems and record keeping in the small- and medium-sized aquaculture operations that make up the bulk of the sector in Atlantic Canada is not advanced enough to achieve third party certification under most existing or forthcoming standards. The necessity of implementing such a system and having it verified by a consultant auditor would place a heavy financial burden upon these businesses. This project seeks to address that technical gap and alleviate the cost by using the Research and Development Coordinator (RDC) as trained technical resources, available to association members at little or no cost.
It is the mandate of the RDCs to act as scientific and technical resources for association members. The project aimed at training RDCs in ISO 14001 Environmental Management Systems and certification schemes currently available to the industry. ISO 14001 is a set of international standards for the development of environmental management systems and supporting audit programs; and is the standard upon which most third party certification schemes are based. This ISO based training will provide the RDCs with the expertise to assist Industry in introducing the necessary paperwork systems, and then be able to assess or audit them against a variety of certification standards prior to their actual certification, to discover the “gaps” that need to be addressed.
Apr. 2011 – Mar. 2012
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: Province of Prince Edward Island; Province of Nova Scotia; Province of Newfoundland
project lead: Peter Warris (Prince Edward Island Aquaculture Alliance)
project team: Peter Warris (PEIAA); Danielle Goodfellow (AANS); Darrell Green (NAIA)
collaborators: Global Trust Consultants
Contact: rd@aquaculturepei.com
www.aquaculturepei.com
Development of a comprehensive fish waste utilization system that produces two products: nutraceutical fish oil and organic fish soil amendment
West Coast Fishculture Ltd. (WCF), located at Lois Lake, BC, has a Steelhead Trout, Oncorhynchus mykiss, production facility consisting of a hatchery, a grow-out system and a processing plant. Prior to this project, WCF identified several options for recovering valuable products from fish farming and processing wastes. This project developed those ideas from concept to commercialization. It makes WCF a unique aquaculture operation through its efforts to try to productively utilize 100% of the fish it produces, head, guts, and all.
This waste utilization system delivers the following benefits: 1) the system consistently processes 100% of all waste streams; 2) the system removes high grade fish oil which may be used as fuel, and soon may be approved for nutraceutical applications; and 3) the system converts all remaining waste to soil amendment which has achieved organic certification providing increased value in the market place.
The system is an economically-viable alternative to current methods for disposal of aquaculture wastes such as composting. The WCF waste management system produces value added products from previous waste streams in an environmentally friendly and economically feasible manner.
This demonstration project is a showcase for sustainable fish farming with a 100% waste utilization system and as such is an important step forward for aquaculture sustainability.
Apr. 2011 – Mar. 2012
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: Agri Sea Biodiesel; CPI Pumps & Irrigation Inc.; Canadian Aqua Start; Little Wing Oysters Ltd.; Mac’s Oysters Ltd.; Nelson Island Sea Farms Ltd.; Taylor Shellfish Canada ULC
project lead: Bill Vandevert (West Coast Fish Culture Ltd.)
project team: Bill Vandevert, Bill Ferris, John Christie, Ward Griffioen (West Coast Fish Culture Ltd.); Joan McKay (J. McKay Aquatech)
Contact: Westcoastfishculture@shaw.ca
http://simplyfish.ca/
Impacts of shellfish aquaculture on marine vegetation
Marine vegetation, such as seagrass and seaweeds, form the foundation of many nearshore ecosystems and are considered critical habitat for many ecologically and economically important species. Shellfish aquaculture has the potential to impact marine vegetation in a variety of ways: via waste particles smothering vegetation, increasing water clarity affecting light penetration (thereby enhancing growth of marine vegetation), and eutrophication (fueling growth of epiphytes that compete with the seagrasses). The range of effects of shellfish culture on marine vegetation can be complex; however, our understanding of the interactions between them is limited.
Baynes Sound, BC, is an area of intensive shellfish aquaculture and is therefore an ideal location for this research. In addition, data on the oceanography, plankton, and benthic communities in Baynes Sound have been collected for several years. This project will evaluate changes in marine vegetation and associated communities by measuring the relative biomass of each trophic level (grazing, predatory, invertebrates, and fish) along a gradient of effects from intensive shellfish aquaculture. This approach will provide a quick assessment of ecosystem-level effects that may be induced from shellfish aquaculture activities.
Apr. 2012 – Mar. 2013
Funded by: DFO – Program for Aquaculture Regulatory Research (PARR)
project lead: Hannah Stewart (DFO)
project team: Terri Sutherland, Beth Piercey (DFO)
collaborators: Steve Katz (NOAA)
Contact: Hannah.Stewart@dfo-mpo.gc.ca

BC Environmental Management Code of Practice
In the rapidly evolving aquaculture industry and with the recent management change from the provincial to the federal government, the BC Shellfish Grower’s Association (BCSGA) has recognized the need to bring the 2001 Environmental Management Code of Practice (EMCP) up-to-date. The first EMCP was finalized in 2001 and although a good piece a work with a lot of merit, there have been some major changes to the industry nationally over the last decade that now need to be addressed by an updated EMCP. Working with shellfish growers, industry associations, government regulators, and other stakeholder groups, this plan will encompass the best practices available and give farmers the tools to compete in a global economy while preserving the values of Canadian sustainability as a responsible user of our ocean resources.
The final results and template from this project will be shared Canada-wide. This will enable a standardized environmental code of practice for shellfish farming that that has been generated at the grass-roots level and will stand up to subsequent scrutiny.
Apr. 2012 – Mar. 2013
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: BCSGA
project lead: Matthew Wright (BCSGA)
project team: Roberta Stevenson (BCSGA)
collaborators: Fanny Bay Oysters; Pentlatch Seafoods; Odyssey Shellfish; Georgia Straight; CAIA; Mac’s Oysters; Lucky 7 Oysters; Island Sea Farms; Little Wing Oysters; Island Scallops
Contact: matt@bcsga.ca
The historical and social dimensions of salmon aquaculture science
Salmon aquaculture has been a focus of environmental research for over two decades. In this project I am applying the tools of environmental history and science and technology studies to understand how this research has developed, and the roles it has played in public discussions regarding the industry. Several more specific objectives are also being pursued.
First, I am writing an environmental history of salmon aquaculture science. This history will explore the relations between scientific research and the evolving environmental, social, and political dimensions of the industry.
Second, I am examining how the diverse institutions engaged in environmental research — governments, universities, industry, and public interest organizations — have shaped research priorities, research results, and the application of these results.
Third, I am investigating the movement of scientific knowledge of salmon aquaculture between research sites in Canada, Norway, Ireland, and Scotland.
Fourth, I am examining the prospects for effective science, which is able to contribute to resolution of controversies regarding this industry.
While this project is examining the full range of environmental science relating to salmon aquaculture, a special focus is on research relating to sea lice.
June 2007 – July 2014
Funded by: Social Sciences and Humanities Research Council of Canada (SSHRC) co-funded by: Genome British Columbia
project lead: Stephen Bocking (Trent U.)
Contact: sbocking@trentu.ca
people.trentu.ca/sbocking/Bocking-Salmon.html

Supporting and advancing key Canadian aquaculture standards and certification initiatives
The purpose of this Canadian Aquaculture Industry Alliance (CAIA)-led project is to test the development of a credible and relevant certification model for application across interested sectors of the Canadian aquaculture industry, assuring buyers and consumers that their farmed seafood has been produced in an environmentally responsible manner, and with best management practices for food safety and quality. The model intends to comprehensively measure industry and farm level performance to allow a certification claim of Responsible Aquaculture Management against established FAO-based criteria.
The past CAIA projects funded by the Aquaculture Innovation and Market Access Program (AIMAP) have successfully promoted understanding, dialogue, and capacity building for all participating stakeholders, and, in particular, have greatly accelerated Canadian industry preparedness for the adoption of third-party certification programs. These projects are directly supportive of CAIA’s continuing efforts to build a record of Canadian excellence in these important market access subjects. With support through the AIMAP program, CAIA is pleased to have been able to provide assistance to member companies interested in continuous improvement and third party certification. Past benchmarking projects have been directly relevant to current activities and especially relevant for supporting CAIA’s initiative to test the FAO-based Responsible Aquaculture Management certification model.
Apr. 2012 – Mar. 2013
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: Newfoundland Aquaculture Industry Association (NAIA); Northern Ontario Aquaculture Association (NOAA); PEI Aquaculture Alliance (PEIAA); BC Salmon Farmers Association (BCSFA); Atlantic Canada Fish Farmers Association (ACFFA)
project lead: Ruth Salmon (CAIA)
project team: Dave Garforth, Cormac O’Sullivan, Peter Marshall (Global Trust Certification Ltd.); Derek Leebosh,(Environics Research)
collaborators: Canadian Aquaculture Standards Forum Committee Members (CASF)
Contact: ruth.salmon@aquaculture.ca
Developing Camelina as the next Canadian oilseed
Camelina sativa is an ancient plant that is being explored as a partial replacement of fish meal and fish oil in aquaculture feed. The Camelina Project is a cross-Canada project that is looking at many aspects and uses of the plant, with the Atlantic region focusing on the aquaculture applications.
The Project is assessing the effects of Camelina in the diets of finfish based on fish performance and health. Digestibility trials on diets containing camelina by-products have been completed in Atlantic Cod, Rainbow Trout, and Atlantic Salmon. Feeding trials using different inclusion levels of Camelina meal and/or oil have been completed in cod and Rainbow Trout, and a salmon trial started in August 2012. Results from the feeding trials have demonstrated that trout and cod can tolerate full or partial substitutions of fish oil for Camelina oil, and can tolerate various levels of Camelina meal inclusion. Lipid biochemistry and genomics analyses of fish tissues from these feeding trials are ongoing.
The project is progressing well towards providing information on the performance of fish that are fed Camelina-containing diets. This information can be used to inform the aquaculture industry of the optimal levels of Camelina inclusion in fish feed.
June 2010 – June 2014
Funded by: Atlantic Canada Opportunities Agency – Atlantic Innovation Fund co-funded by: Agriculture and Agri-Food Canada – Agricultural Bioproducts Innovation Program; Atlantic Oilseeds; Colorado State University; Giessen University; Genome Atlantic; Genome Prairie; Memorial University of Newfoundland – Ocean Sciences Centre; Minas Seed; Dalhousie Faculty of Agriculture; Nova Scotia Agricultural College; Province of Nova Scotia – Department of Agriculture/Department of Fisheries and Aquaculture; Province of New Brunswick – Department of Agriculture and Aquaculture; Province of Saskatchewan – Ministry of Agriculture; Saskatchewan Canola Development Commission; The Research and Development Corporation of Newfoundland and Labrador; Cooke Aquaculture
project lead: Isobel Parkin, Claude Caldwell
project team: Matt Rise, Chris Parrish (MUN); Derek Anderson (Dalhousie U.); Dwayne Hegedus (Agriculture and Agri-Food Canada (AAFC)
Contact: sking@genomeatlantic.ca
www.camelinaproject.ca

FishProbio: a sustainable and effective alternative strategy for preventing major opportunistic infections in salmonids
In Canada, the booming freshwater aquaculture industry is dominated by salmonid production. Mass production favours the onset of disease, which leads to huge economic losses. However, the use of antibiotics to control bacterial diseases is raising serious concerns because antibiotics persist in organisms and the environment and affect the selection of resistant pathogens. There is an urgent need to develop safe, effective prevention and treatment strategies, while maintaining the industry’s economic viability. The probiotic approach clearly meets these criteria. We chose the Brook Trout (Salvelinus fontinalis) as a model, as it is the most widely harvested species in Quebec. Our experimental strategy is to collect bacteria naturally present in the skin mucus of healthy fish. The initial results of our approach show that seven bacteria from the fish skin microbiome are strong competition in vitro against two opportunistic pathogens (Flavobacterium columnare and F. psychrophilum), making those very promising probiotic candidates. We successfully tested the curative efficacy of the best probiotic candidate in an in vivo experiment. Infected fish were treated by adding the probiotic on a daily basis. The treatment improved fish survival by 54 to 86% compared to fish in control ponds (infected fish not treated with probiotics).
Jan. 2008 – Dec. 2012
Funded by: NSERC – Strategic Subvention; Société de recherche et de développement en aquaculture continentale (SORDAC); RAQ; Aquaculture Forestville; NSERC – Collaborative Research and Training Experience Program (CRTEP)
project lead: Nicolas Derome (U. Laval)
project team: Sébastien Boutin, Louis Bernatchez (U. Laval); Céline Audet (UQAR)
collaborators: Fran Hansen (PEI Shellfish Association)
Contact: nicolas.derome@bio.ulaval.ca
Development of a biodiesel production process from microalgae using cheese whey permeate
Reducing the cost of microalgae raw oil could allow the advent of a new fossil fuel competitor on the market and this would help limit the amount of carbon dioxide (CO2) emitted by mankind into the atmosphere. This must be initiated by the elaboration of new industrial processes allowing the mass production of microalgae oil at lower costs. While the avenue of microalgae biomass production in combination with wastewater remediation has been identified as a cheap route for producing biomass, many factors significantly impair growth and productivity. The aim of the present work is to evaluate another avenue, using a major co-product from the cheese industry, whey, for use in a microalgae-based biodiesel production process. A quick pre-treatment of whey can be realized to produce a concentrated and purified lactose stock, using already available industrial equipment in this industry. Promising results on the use of a selected microalgal strain to grow on lactose in mixo/heterotrophic conditions are presented, as part of an eventual industrial process that would convert it into microalgal oil. Cellular lipid profiles of the microalgae at different growth stages are also investigated to ensure the production of a biodiesel with adequate properties.
Sept. 2010 – Jan. 2014
Funded by: Fonds de recherche Nature et technologies du Québec (FQRNT); RAQ
project lead: Jean-Michel Girard (U. Sherbrooke)
project team: Nathalie Faucheux, Michèle Heitz (U. Sherbrooke); Réjean Tremblay, Jean-Sébastien Deschênes (UQAR)
collaborators: Fran Hansen (PEI Shellfish Association)
Contact: Jean-Michel.Bergeron.Girard@USherbrooke.ca
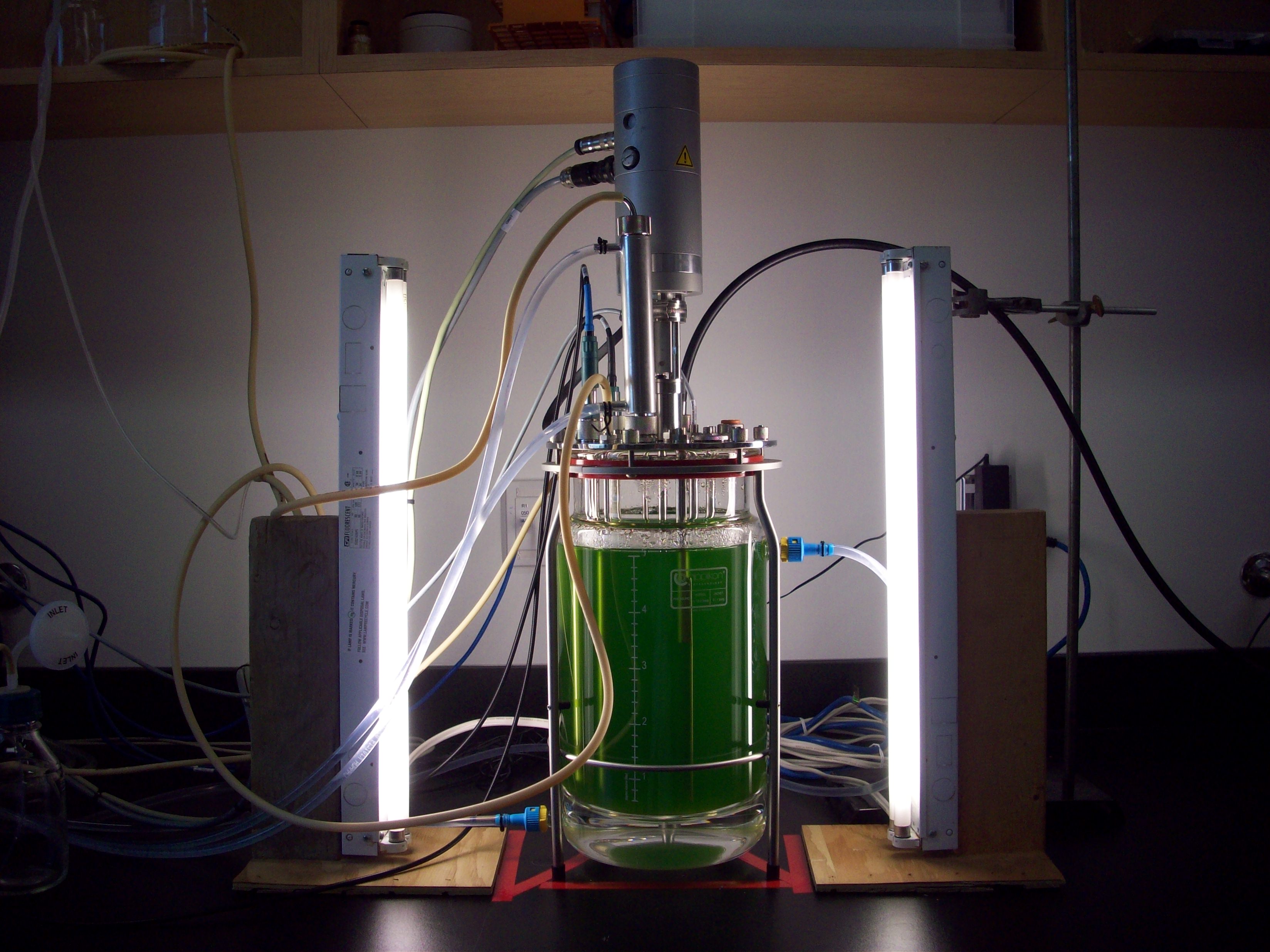
Novel antifouling technologies
The Centre for Biofouling Research at St. Francis Xavier University is a multidisciplinary team researching cost effective and environmentally friendly solutions to manage biofouling. Our goal is to design and demonstrate the effectiveness of biofouling resistant surfaces for submerged surfaces in the ocean, with a particular focus on reducing fouling in cold temperate waters. Our team, which includes expertise on the molecular dynamics of biofilms, microorganisms, biomechanics, marine larval biology, and field experimentation, is using a range of techniques to explore both chemical and physical means to either reduce development or promote release of fouling communities. We are currently focusing on deterrents for invasive tunicates (sea squirts).
Apr. 2011 – Mar. 2013
Funded by: Encana co-funded by: NSERC
project lead: Truis Smith-Palmer (StFX)
project team: Cory Biship, Edwin DeMont, Darren Derksen, Lori Graham, David Pink, Russell Wyeth (StFX)
Contact: tsmithpa@stfx.ca
sites.stfx.ca/biofouling/

Development of Feed for Culturing Lobster Larvae for Seeding in a Natural Environment
Since 2002, Homarus Inc., a not-for-profit organization managed by the Maritime Fishermen’s Union (MFU), has been collaborating with the Coastal Zones Research Institute Inc. (CZRI) to ensure the production of post-larval lobster for seeding in a natural environment. Current aquaculture techniques produce over 100,000 post-larvae per year. However, larvae production costs remain relatively high. To date, no feed is perfectly suited to the culture of American Lobster larvae. As a result, hatchery-produced lobster larvae are fed with a tri-mix diet made up of frozen Brine Shrimp, commercial Brine Shrimp flakes, and ArteMac™. However, these products are expensive, difficult to obtain (requiring importation) and must be kept frozen until they are used. There is therefore an imminent need to develop a dry, practical diet for lobster larvae. The objective of this research is therefore to develop a dry feed for lobster larvae that would meet their nutritional needs. Producing such a feed would allow and greatly facilitate the implementation of lobster larvae-raising techniques within the industry.
APR. 2010 – MAR. 2012
Funded by: NSERC-CRD co-funded by: Homarus Inc.; UMCS; UMCM; CZRI
project lead: Sébastien Plante (UMCS)
Project team: Marc Surette (UMCM)
collaborators: Martin Mallet (Homarus Inc.); Maxime Boudreau (UMCM); France Béland (CZRI)
Contact: sebastien.plante@umoncton.ca

Anaerobic digestion of fish offal and sawdust
Anaerobic digestion is an attractive option for manure/waste management because of its potential to digest agricultural and industrial residues, while reducing greenhouse gas emissions, mitigating pathogens and odours, and increasing ionized nutrients in the material being digested. This study will test this option by investigating the digestion of fish offal and sawdust using 20-L digesters (microorganisms that break down biodegradable material) under two operational strategies. The digester studies will focus on optimizing biogas/methane production by changing organic loading rates, ratio of fish offal to sawdust, and feeding technique (batch vs semi-continuous). The biogas/methane yields determined in this study will be used to assess the economic and performance feasibility of a full scale system.
Dec. 2011 – Mar. 2013
Funded by: DFO – Aquaculture Collaborative Research and Development Program (ACRDP) co-funded by: Meeker’s Aquaculture
project lead: Doug Geiling (DFO)
project team: Richard Moccia, David Bevan, Anna Crolla (U. Guelph)
collaborators: Mike Meeker (Meeker’s Aquaculture)
Contact: Doug.Geiling@dfo-mpo.gc.ca
Assessing the bioavailability of methionine and lysine from different sources
Supplementing synthetic methionine (Met) and/or lysine (Lys) to diets of terrestrial farm animals in order to meet the nutritional requirements for methionine or lysine is a common practice. Different forms of supplemental Met and Lys are produced and commercialized by different manufacturers. Limited information exists on the bioavailability of the different forms of Met and Lys to fish species.
The project involves two separate dose-response feeding trials, in which graded levels of test synthetic amino acids, three methionine sources and two lysine sources, are fed to juvenile Rainbow Trout for 12 weeks. Relative bio-efficacy of test amino acids is to be assessed using a slope-ratio assay method.
May 2012 – ongoing
Funded by: Evonik Industries AG, Germany co-funded by: OMAFRA
project lead: Christopher Powell, Dominique P. Bureau (U. Guelph)
project team: Kabir Chowdhury (U. Guelph)
collaborators: Andreas Lemme (Evonik Industries AG)
Contact: dbureau@uoguelph.ca, cpowell@uoguelph.ca
Physiology of triploid fish
Triploidy is the only management tool currently available for ensuring reproductive sterility of farmed fish. Sterile populations can be of direct benefit to industry, since sexually mature fish often have reduced flesh quality and poor disease resistance. Sterility also addresses the risk of escaped fish breeding in the wild. However, triploids are rarely used in aquaculture because of performance limitations. Triploid red blood cells (RBCs) are 40-50% larger than diploid RBCs. We are investigating whether this change in cell size, and the associated reduction in cellular surface area per unit volume, affects triploid performance. For instance, increased size may affect RBC passage through narrow capillaries, and reduced surface area may limit ion transfer or respiratory gas exchange across the cell membrane. We are therefore examining the ability of triploid RBCs to maintain their basic structure and ion transport capabilities in vitro, and also conducting in vivo experiments to look at RBC flow under various conditions of thermal and hypoxic stress. We have adopted Zebrafish as a model species for some of this research because of the availability of stocks with fluorescent RBCs and endothelial linings of their blood vessels. The research will then be extended to Atlantic Salmon.
sept. 2012 – ongoing
Funded by: NSERC co-funded by: New Brunswick Innovation Foundation (NBIF)
project lead: Tillmann Benfey (UNB)
Project team: Chris Small, Nicole Nader, Bryan Crawford (UNB)
Contact: benfey@unb.ca
www.unb.ca/fredericton/science/biology/Faculty/Benfey.html
Protective barrier to counter Sea Otter predation
Currently there are very little data on methods to reduce the impact of Sea Otter predation on shellfish farm stocks, as this is a new issue in most aquaculture farm areas on the west coast of Canada. This predation will become more problematic as the otters expand their range into more populated areas of the coast. As an example, stock losses would likely be 80 to 85 percent of standing stock on a Manila Clam farm without protection. Obviously, this would not be viable on a commercial basis.
Nootka Sound Shellfish Ltd. will develop, test and evaluate novel protective barriers to counter Sea Otter predation of Manila Clams which is a serious threat on the North Coast and North Island of the Pacific Region. Experts indicate that this is an increasing and significant challenge for shellfish farmers. Sea Otters eat up to 40% of their body weight daily so a growing population is threatening the long term success of co-located shellfish farms. To respond to this threat, Nootka Sound Shellfish will be designing and demonstrating a two net system using nylon and polypropylene to protect their farm from Sea Otter predation, to achieve clear productivity gains through limiting Sea Otter predation and to maintain the safety of the Sea Otters.
Apr. 2012 – Mar. 2013
Funded by: DFO – Aquaculture Innovation and Market Access Program (AIMAP) co-funded by: Pacific Net & Twine; Summer Breeze Aquaculture Products; Harbour Chandler
project lead: Kevin Vautier (Nootka Sound Shellfish Ltd.)
project team: Kevin Vautier (Nootka Sound Shellfish Ltd.)
collaborators: BC Shellfish Growers Association; MV Uchuck III
Contact: nss@island.net
A meta-analysis of essential amino acid requirements in fish
Aquaculture feeds are formulated to promote animal’s growth and health, while minimizing feed costs and environmental impacts. Achieving maximum efficiency implies a precise knowledge of nutritional requirements, including essential amino acids (EAA). Several reviews have identified great variability in EAA requirement estimates across and within fish species. The construction of a standardized database using information from published studies on EAA requirements of fish and a meta-analysis using this database were the focus of this study. The primary objectives were to estimate EAA requirement of different fish species and identify sources of variability in the estimates of requirement. Over 250 studies on EAA requirement of fish were identified. However, fewer than 25% met the quality criteria required for the database and meta-analysis. The dataset also revealed the high fragmentation of our knowledge in terms of EAA requirement with a few commercially important species represented and limited amount of work on many of the EAA. The meta-analysis revealed the critical importance of proper design of experimental design. Numerous trials did not presenting a clear dose-response and did not allow accurate requirement estimation. Finally, this study also indicates that it is paramount to report basic information such as live weight, experiment duration, temperature, and composition of the diet. Without these parameters, results cannot be standardized across studies, thus resulting in a loss of knowledge.
Aug. 2009 – Dec. 2011
Funded by: NSERC
project lead: Guillaume P. Salze, Dominique P Bureau (U. Guelph)
collaborators: Margaret Quinton (U. Guelph)
Contact: dbureau@uoguelph.ca

- Date modified: