Unidentified Species of Bonamia from Various Oysters and Locations
On this page
Category
Category 3 (Host Not in Canada)
Common, generally accepted names of the organism or disease agent
Microcell disease, Bonamiasis, Bonamiosis, Haemocyte disease of oysters.
Scientific name or taxonomic affiliation
Bonamia spp. which resemble Bonamia ostreae a pathogen of European flat oysters Ostrea edulis, Bonamia exitiosa a pathogen initially described from New Zealand dredge oysters Ostrea chilensis but now known to occur in several species of oysters from around the world, Bonamia roughleyi a pathogen originally described from Sydney rock oysters Saccostrea glomerata but currently of questionable identity, and Bonamia perspora a parasite of the crested or horse oyster Ostrea stentina (=Ostreola equestris), have been reported from various species of oysters in distant locations. Hall et al. (2010a, b) indicated that at least some of the Bonamia spp. isolates from Australia, Chile, Argentina, and the eastern USA have genetic affinities to the Bonamia exitiosa/Bonamia roughleyi clade. Prior to 2014, it was not known whether these Bonamia exitiosa-like microcells consists of different strains of one species or the group consists of a multiple of species (Lormann et al. 2009). Also, for most of the microcells in this B. exitiosa-like group, relative few data on histology, ultrastructure and molecular sequences had been available and not sufficient to unequivocally discriminate between species (Hill et al. 2010b). Recent molecular analysis of the sequence of one gene, specifically, segments of the small subunit (SSU or 18S) and internal transcribed spacer (ITS1-5.8S-ITS2 which make up the ITS rDNA locus) regions of the ribosomal DNA gene complex, revealed that many of the isolates from various locations were B. exitiosa (Engelsma et al. 2014, Hill et al. 2014). The information presented below is a listing of all microcells initially reported as Bonamia sp. and more recent interpretations of their identity. Each reported geographic location and corresponding host species was assigned a letter code which is consistently applied to all other information pertaining to the organism under each of the following headings.
Geographic distribution
- Western Australia, Victoria, New South Wales and Tasmania, Australia (Hine and Jones 1994, pers. comm. Ben Diggles Ph. D., DigsFish Services).
- Chiloe and Quihua Islands, Southern Chile (Kern 1993, Campalans et al. 2000, Lohrmann et al. 2009, Campalans and Lohemann 2009).
- San Antonio Bay and adjacent areas in San Matías Gulf, North Patagonia, Argentina (Kroeck and Montes 2005, Kroeck et al. 2008, Hill et al. 2014).
- East coast of the USA including Bogue and Masonboro Sounds, North Carolina (Burreson et al. 2004; Carnegie et al. 2005, 2006; Bishop et al. 2006; Wilbur et al. 2008), York River (Gloucester Point) and Chesapeake Bay (Schott et al. 2008).
- Hawaii (Hill et al. 2008, 2014).
- Tomales Bay, California, USA (Hill et al. 2014).
Host species
- Ostrea angasi. The identity of the Bonamia is likely to be B. exitiosa (Engelsma and Hine 2009a, Hill et al. 2010b).
- Ostrea (=Tiostrea) chilensis. Genetic analysis by Hill et al. (2014) indicated that the Bonamia sp. in O. chilensis from Chile appeared in the B. exitiosa clade in SSU rDNA phylogenetic analyses, but diverged from this clade in the ITS rDNA analyses. Thus, Hill et al. (2014) and Engelsma et al. (2014) indicated that including the parasites from O. chilensis in Chile within B. exitiosa may not be justified, and this Bonamia sp. may represent a novel species.
- Ostrea puelchana and Ostrea stentina. The Bonamia in these oysters have now been identified as B. exitiosa (Oehrens Kissner et al. 2014, Hill et al. 2014).
- Crassostrea ariakensis, Ostrea stentina (=Ostreola equestris) and probably Crassostrea virginica. Genetic analysis indicated that the Bonamia sp. in C. ariakensis and O. stentina was B. exitiosa (Dungan et al. 2012, Audemard et al. 2014). Note that B. exitiosa was detected in the same species, O. stentina, as Bonamia perspora described by Carnegie et al. (2005, 2006). Although infection was not initially detected in wild Crassostrea virginica from the same location as infected, experimentally introduced, C. ariakensis (Carnegie et al. 2006), Wilbur et al. (2008) detected Bonamia sp. DNA (via quantitative real-time PCR assay) in C. virginica from three estuaries in south eastern North Carolina with no detectable impact on the health of the positive oysters. Subsequent assays indicate that B. exitiosa occurs in C. virginica in the eastern USA (Engelsma et al. 2014, Hill et al. 2014, and see OIE Reports 2012 & 2013 in References below). In situ hybridisation (ISH) was used to detect a parasite closely related to B. exitiosa in archival histological materials of O. edulis from Chincoteague Bay, Virginia (Hill et al. 2014).
- Dendostrea (=Ostrea) sandvicensis. The small subunit ribosomal DNA (SSU rDNA) sequence from the putative Bonamia sp. was unique with only 90-91% similar to published sequences from other Bonamia spp. (i.e., B. exitiosa, B. ostreae, and B. perspora) (Hill et al. 2014). Although two specifically designed, fluorescently labeled ISH probes hybridized with the Bonamia sp. from D. sandvicensis, B. exitiosa-specific probes did not (Hill et al. 2014). Also, internal transcribed spacer (ITS) ribosomal region based phylogenetic analyses indicated that the parasite of D. sandvicensis in Hawaii was a distinct species of Bonamia (Hill et al. 2014, Engelsma et al. 2014).
- Ostrea edulia. The Bonamia sp. in O. edulis from Tomales Bay, California in 2005 was closely related to both B. exitiosa and Bonamia sp. from O. chilensis in Chile (Hill et al. 2014). Specifically, Hill et al. (2014) reported that the SSU rDNA region was 99-100% similar between the isolates but there was only 83-86% ITS rDNA sequence similarity between B. exitiosa and the isolates from Chile. Hill et al. (2014) concluded that inclusion of this parasite within B. exitiosa may not be justified, and this lineage may represent a novel species. Note that Bonamia ostreae is also known to occur in O. edulis from California (Hill et al. 2014).
Impact on the host
- Like B. ostreae and other B. exitiosa, this intrahaemocytic protozoan quickly becomes systemic with overwhelming numbers of parasites coinciding with the death of the oyster. The B. exitiosa in O. angasi from Tasmania causes a very different pathology than B. exitiosa in Ostrea chilensis from New Zealand by being epitheliotropic and associated with, but rare in, focal abscesses (Engelsma and Hine 2009a). However, Heasman et al. (2004) observed Bonamia sp. only in haemocytes and usually in areas of haemocytosis in O. angasi from New South Wales. Hine and Jones (1994) speculated that the Australian Bonamia sp. maybe were derived from a common ancestor of B. exitiosa at the end of the Cretaceous, when New Zealand separated from Victoria, Australia or this parasite may have evolved in New Zealand and following mass mortalities of Australian oysters in the late 19th century was introduced to Australia with New Zealand oysters that were laid in Victorian and Tasmanian waters for the restaurant trade.
- Significance is unknown. Although high “levels” of infection were noted by Kern (1993) and prevalence up to 20% was detected by Arzul et al. (2005), no significant mortalities have been reported. Campalans and Lohrmann (2009) reported relatively low prevalence (up to 6%) from southern Chile with the lowest prevalence (2%) observed in April (autumn). The presence of the Bonamia sp. always involved severe haemocytic infiltration in the connective tissue of the mantle, gills and digestive gland (Campalans and Lohrmann 2009).
- This Bonamia sp. was associated with high mortalities (33% after 18 months, 80% after 31 months and 95% when the O. puelchana reached market size after 32 to 36 months of culture) and resulted in the termination of the culture effort (Kroeck and Montes 2005). Kroeck et al. (2008) proposed that Bonamia sp. is an enzootic parasite of San Matías Gulf, because of its occurrence in native populations of O. puelchana from natural dense beds located close to the impacted oyster culture facilities. In the natural beds, there were no differences between the shell length of the parasitized and non-parasitized oysters and the probability of infection was independent of oyster sex (Kroeck et al. 2008). Assessment of the status of B. exitiosa in natural populations of O. puelchana 14 years after the epizootic indicated that oyster density was markedly decreased and oyster mean size was lower, indicating that bonamiosis controlled the population structure of the oysters in the persisting oyster beds during this period (Oehrens Kissner et al. 2014).
- High prevalence of infection (up to 100%) were associated with high mortalities among triploid C. ariakensis in experimental field trials within a month of being transferred to grow-out locations in Bogue Sound, North Carolina in the summer of 2003. Cohorts at a distant location in North Carolina (Pamlico Sound) and in Chesapeake Bay, Virginia did not experience mortalities and were not infected with the Bonamia sp. (Burreson et al. 2004). However, Schott et al. (2008) reported a low prevalence of infection (2.7%) in triploid C. ariakensis experimentally reared near the mouth of the York River, Chesapeake Bay. Because C. ariakensis is not native to the east coast of the United States and was known to be free of infectious pathogens prior to the field trials, the source (natural host) for this Bonamia sp. is unknown. However, this Bonamia sp. was detected in low prevalence (3.3%) in O. equestris from Bogue Sound, North Carolina in June 2003. Experimental results indicate that salinity below 30 parts per thousand were detrimental to Bonamia sp. in C. ariakensis (Audemard et al. 2005, 2008a) and warm temperature (greater than 20 °C) was associated with higher host mortality than colder temperatures, suggesting that temperature influenced Bonamia sp. pathogenicity (Audemard et al. 2008b). However, Bonamia sp. may be able to persist in C. ariakensis under a combination of low temperature and meso- to euhaline salinities (Audemard et al. 2008b). Also, Bonamia sp. activity was found to be seasonal with oyster mortality reaching 100% in late spring and summer deployments, early fall deployments showed reduced (17–82%) mortality and late fall and early winter deployments (made at temperatures less than 20 °C) did not develop Bonamia sp. infections (Carnegie et al. 2008). Crassostrea ariakensis can acquire infection with Bonamia sp. after 2 weeks of exposure in enzootic areas of North Carolina and laboratory studies suggest that this parasite can be directly transmitted between C. ariakensis in the laboratory (Audemard et al. 2008c). Note that this parasite was subsequently identified as B. exitiosa (Audemard et al. 2014, Engelsma et al. 2014, Hill et al. 2014).
- and f. None reported.
Diagnostic techniques
Engelsma and Hine (2009) indicated that PCR, heart imprints, and histology can be used for targeted surveillance and presumptive diagnosis, and DNA sequencing is the recommended method for confirmatory diagnosis. Engelsma et al. (2014) presented a summary and discussion of the various diagnostic techniques to detect Bonamia spp.
Tissue Imprint (“Heart Smears”)
Make acetone- (or methanol-) fixed impression smears from heart tissue (preferably ventricle since the auricles contain an abundance of serous cells which make detection of the parasite difficult). Stain with Wright, Wright-Giemsa or equivalent stain (e.g. Hemacolor, Merck; Diff-QuiK, Baxter). Examine for 2-5 µm spherical or ovoid organisms (microcells) with a central nucleus (fried egg appearance) within or outside the haemocytes.
The organisms are enlarged by this method compared to those in fresh or histological preparations.
Histology
- Examine haematoxylin and eosin stained tissue cross-sections for intracellular microcells (2-3 µm in diameter) within haemocytes. Bonamia are distributed systemically in advanced infections. In early infections, Bonamia are often observed within haemocytes in focal infiltrations in the connective tissue of the gill and mantle, and in the vascular sinuses around the stomach and intestine.
- and d. Not reported
- Cells of Bonamia sp. were observed in connective tissue (free or within haemocytes) of the gills and around the digestive gland, stomach, intestine and gonad. Gross signs, histopathological alterations in O. puelchana, and Bonamia sp. cytological morphology resemble those reported for B. exitiosa (Kroeck 2010).
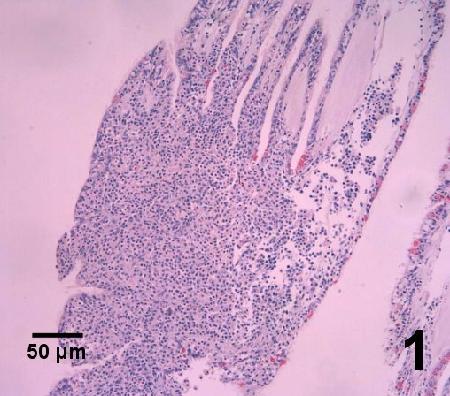
Figure 1. Accumulation of haemocytes within the gills of Ostrea angasi infected with Bonamia sp. in Australia. Image provided by Ben Diggles Ph. D., DigsFish Services.
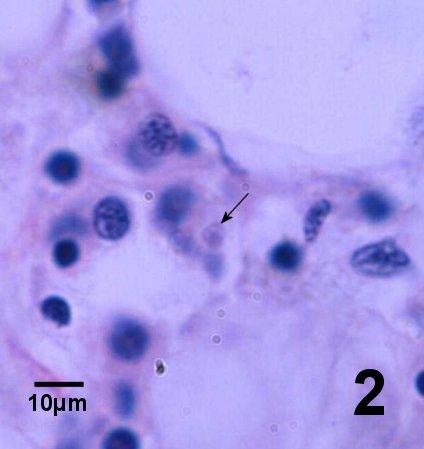
Figure 2. One Bonamia sp. (arrow) within a haemocyte among a group of haemocytes between vesicular connective tissue cells in a subclinical infection in Ostrea angasi. Image provided by Ben Diggles Ph. D., DigsFish Services.
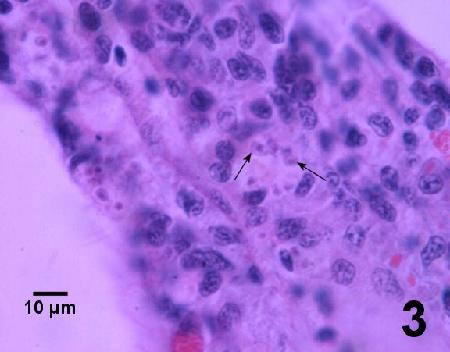
Figure 3. A cluster of Bonamia sp.(arrows) in a haemocyte involved in an accumulation of haemocytes within the gills of a diseased Ostrea angasi. Image provided by Ben Diggles Ph. D., DigsFish Services.
- and f. The histological presentation of the parasite in each host was typical of infection by ‘microcell haplosporidian' Bonamia spp. as described by Carnegie and Cochennec Laureau (2004). The parasite cells were generally intrahaemocytic, sometimes extracellular where haemocytes had lysed, with no presentation of more conventionally haplosporidian forms such as spores or large multinucleate plasmodia (Hill et al. 2014). Infections were light to moderate in intensity and light to moderate haemocytosis was typically observed as a host response (Englesma et al. 2014). Disruption of tissue was only modest (Hill et al. 2014).
Electron Microscopy
- The ultrastructure of Bonamia from Ostrea angasi from Australia appears conspecific with B. exitiosa (Hine et al. 2014).
- Lohrmann et al. (2009) and Campalans and Lohrmann (2009) reported on the ultrastructure of the Chilean Bonamia sp. and indicated that: 1) it resembles B. ostreae in size, the low number of mitochondrial profiles, and the prevalence and mean number of lipid droplets; 2) it differs from B. ostreae in the greater prevalence of nuclear membrane-bound Golgi (NM-BG), associated haplosporogenesis, and smaller size of haplosporosomes; 3) it resembles B. exitiosa in the number of haplosporosomes, prevalence of lipid droplets, anastomosing endoplasmic reticulum and NM-BG, presence of circles of smooth endoplasmic reticulum, and confronting and cylindrical cisternae; 4) it also appears to have a similar developmental cycle to B. exitiosa with larger forms occurring in winter (August). Based on similarities in ultrastructure and developmental stages between B. exitiosa and the Chilean Bonamia sp., Lohrmann et al. (2009) suggested that the 2 species are related, and that the Chilean Bonamia sp. is either B. exitiosa, a sub-species of B. exitiosa, or a separate species closely related to B. exitiosa. Hine et al. (2014) indicated that although there were similarities in the development of a larger uni-nucleate stage and the occurrence of cylindrical confronting cisternae, differences were noted between B. exitiosa in O. chilensis from New Zealand and the Chilean Bonamia in the same species of oyster from Chile. Specifically, the Chilean Bonamia is smaller with fewer, smaller haplosporosomes. However, this feature does not adequately distinguish it from New Zealand B. exitiosa and the clarification of the identity of Chilean Bonamia must await molecular studies (Hine et al. 2014).
- The ultrastructure of Bonamia from Ostrea puelchana from Argentina appears conspecific with B. exitiosa (Hine et al. 2014).
- The ultrastructure of Bonamia from Crassostrea ariakensis from the east coast of the USA appears conspecific with B. exitiosa (Hine et al. 2014).
- and f. Not reported
DNA Probes
- The sequence of segments of the ribosomal RNA locus which includes parts of the small subunit ribosomal DNA (SSU rDNA or 18S rDNA) gene and the internal transcribed spacer (ITS) regions (consisting of ITS1-5.8S-ITS2) gene was used to confirm that the Bonamia in O. angasi from Australia was B. exitiosa (Corbeil et al. 2006b, Engelsma et al. 2014, Hill et al. 2014). A real-time TaqMan PCR assay was developed for the detection of Bonamia spp. also detected the Bonamia sp. from O. angasi (Corbeil et al. 2006a).
- Partial sequences of the SSU (18S) and internal transcribed spacers (ITS) regions of the ribosomal DNA (rDNA) suggested that the parasite was closely related but distinct from B. ostreae and B. exitiosa (Arzul et al. 2005, White et al. 2008). One conventional PCR assay designed for specific amplification of B. exitiosa (Carnegie et al. 2008), will also amplify DNA of the Bonamia sp. in O. chilensis from Chile (Engelsma et al. 2014). Also, the chromogenic ISH assay designed to detect only B. exitiosa (Hill et al. 2010b) will also detect the sister lineage of Bonamia sp. from Chilean O. chilensis (Engelsma et al. 2014).
- Based on genetic (parsimony) analysis, White et al. (2008) suggested that a single Bonamia species which resembles B. exitiosa may occur in New Zealand, Australia, Argentina and North Carolina. However, Kroeck (2010) proposed to treat the Argentinean species as B. exitiosa-like until more molecular and ultrastructural studies are conducted to determine the correct taxonomy. More recently, Hill et al. (2014) determined that the Bonamia from O. puelchana and Ostrea stentina in Argentina was B. exitiosa based on sequence data of SSU and ITS spacer regions of ribosomal DNA.
- Two polymerase chain reaction (PCR) assays known to amplify the SSU rDNA of various species of Bonamia (Carnegie et al. 2000 and Cochennec et al. 2000) produced amplicons of the predicted size from all infected C. ariakensis that were assayed. Sequencing of the products yielded a single SSU rDNA sequence (GenBank Accession number AY542903) that was clearly a species of Bonamia but different from other Bonamia spp. (B. ostreae, B. exitiosa, and B. roughleyi) for which sequence data was available (Burreson et al. 2004). In situ hybridisation with Bonamia-specific probes reacted with the Bonamia sp. in C. ariakensis from Bogue Sound, North Carolina. Schott et al. (2008) reported novel Bonamia-related sequences as well as those closely related to previously described Bonamia spp. in C. ariakensis from Chesapeake Bay, USA (GenBank Accession number AY923853-AY923857). More recently, Hill et al. (2014) determined that the Bonamia in C. ariakensis and Ostrea stentina from the eastern coast of the USA was B. exitiosa based on sequence data of SSU and ITS spacer regions of ribosomal DNA.
- The Bonamia sp. detected by genus specific PCR and histological examination in D. sandvicensis from Hawaii was found to be novel and basal to the rest of the Bonamia clade based on phylogenetic analysis of the SSU and ITS rDNA gene sequences (Hill et al. 2008, 2014).
- Hybridization with the Bonamia sp. in O. edulis from California was observed via ISH using digoxigenin-labeled, B. exitiosa-specific probes which target SSU rRNA (Hill et al. 2014). Also, phylogenetic analyses of the SSU rDNA sequences indicated that this parasite appeared to be in the B. exitiosa clade. However, the ITS rDNA analyses indicated that the parasite diverged from this clade (Hill et al. 2014). One conventional PCR assay designed for specific amplification of B. exitiosa (Carnegie et al. 2008), will also amplify DNA of the Bonamia sp. in O. edulis from California (Engelsma et al. 2014). Also, the chromogenic ISH assay designed to detect only B. exitiosa (Hill et al. 2010b) will also detect the sister lineage of Bonamia sp. in O. edulis from California (Engelsma et al. 2014).
Methods of control
To date there are no known eradication or control procedures. Until the method(s) of transmission and host specificity of these parasites has been fully investigated, the movement of oysters out of endemic areas should be avoided. Carnegie et al. (2008) determined that infection with Bonamia sp. in Crassostrea ariakensis in North Carolina was seasonal and influenced greatly by temperature. Thus, avoiding peak seasonal Bonamia sp. activity (late spring through early fall when water temperatures are greater than 20 °C) will be essential for culturing C. ariakensis in Bonamia sp.-enzootic waters (Carnegie et al. 2008).
References
Arzul, I., J.-P. Joly, M. Robert, B. Chollet, C. Garcia, L. Miossec, N. Cochennec, N. Carrasco, J. Campalans, M. Campalans and F. Berthe. 2005. Microcells in flat oysters, Ostrea chilensis from Chiloe Island, Chile: a new Bonamia species? Journal of Shellfish Research 24: 639. (Abstract).
Audemard, C., R. Carnegie, N. Stokes, E. Burreson and M. Bishop. 2005. Salinity effects on the susceptibility to and persistence of Bonamia ostreae and Bonamia sp. in Crassostrea ariakensis. Journal of Shellfish Research 24: 639. (Abstract).
Audemard, C., R.B. Carnegie, N.A. Stokes, M.J. Bishop, C.H. Peterson and E.M. Burreson. 2008a. Effects of salinity on Bonamia sp. survival in the Asian oyster Crassostrea ariakensis. Journal of Shellfish Research 27: 535-540.
Audemard, C., R.B. Carnegie, M.J. Bishop, C.H. Peterson and E.M. Burreson. 2008b. Interacting effects of temperature and salinity on Bonamia sp. parasitism in the Asian oyster Crassostrea ariakensis. Journal of Invertebrate Pathology 98: 344-350.
Audemard, C., R.B. Carnegie, K.M. Hill, C.H. Peterson and E.M. Burreson. 2008c. Investigation of Bonamia sp. transmission among, and incident in, Crassostrea ariakensis. Journal of Shellfish Research 27: 986. (Abstract).
Audemard, C., R.B. Carnegie, K.M. Hill, C.H. Peterson and E.M. Burreson. 2014. Bonamia exitiosa transmission among, and incidence in, Asian oyster Crassostrea ariakensis under warm euhaline conditions. Diseases of Aquatic Organisms 110: 143-150.
Bishop, M.J., R.B. Carnegie, N.A. Stokes, C.H. Peterson and E.M. Burreson. 2006. Complications of a non-native oyster introduction: Facilitation of a local parasite. Marine Ecology Progress Series 325: 145-152.
Burreson, E.M., N.A. Stokes and R.B. Carnegie. 2004. Bonamia sp. (Haplosporidia) found in nonnative oysters Crassostrea ariakensis in Bogue Sound, North Carolina. Journal of Aquatic Animal Health 16: 1-9.
Campalans, M. and K.B. Lohrmann. 2009. Histological survey of four species of cultivated molluscs in Chile susceptible to OIE notifiable diseases (Catastro histológico de cuatro especies de moluscos cultivados en Chile susceptibles a enfermedades de declaración obligatoria a la OIE). Revista de Biología Marina y Oceanografía 44: 561-569. (In English, Spanish Abstract).
Campalans, M., P. Rojas and M. Gonzalez. 2000. Haemocytic parasitosis in the farmed oyster Tiostrea chilensis. Bulletin of the European Association of Fish Pathologists 20: 31-33.
Carnegie, R.B. and N. Cochennec-Laureau. 2004. Microcell parasites of oysters: recent insights and future trends. Aquatic Living Resources 17: 519-528.
Carnegie, R.B., B.J. Barber, S.C. Culloty, A.J. Figueras and D.L. Distel. 2000. Development of a PCR assay for detection of the oyster pathogen Bonamia ostreae and support for its inclusion in the Haplosporidia. Diseases of Aquatic Organisms 42: 199-206.
Carnegie, R.B., N.A. Stokes, C. Audemard and E.M. Burreson. 2005. Bonamiasis in the crested oyster Ostrea equestris in North Carolina, USA. Journal of Shellfish Research 24: 644. (Abstract).
Carnegie, R.B., E.M. Burreson, P.M. Hine, N.A. Stokes, C. Audemard, M.J. Bishop and C.H. Peterson. 2006. Bonamia perspora n. sp. (Haplosporidia), a parasite of the oyster Ostreola equestris, is the first Bonamia species known to produce spores. Journal of Eukaryotic Microbiology 53: 232-245.
Carnegie, R.B., N.A. Stokes, C. Audemard, M.J. Bishop, A.E. Wilbur, T.D. Alphin, M.H. Posey, C.H. Peterson and E.M. Burreson. 2008. Strong seasonality of Bonamia sp. infection and induced Crassostrea ariakensis mortality in Bogue and Masonboro Sounds, North Carolina, USA. Journal of Invertebrate Pathology 98: 335–343.
Cochennec, N., F. LeRoux, F. Berthe and A. Gerard. 2000. Detection of Bonamia ostreae based on small subunit ribosomal probe. Journal of Invertebrate Pathology 76: 26-32.
Corbeil, S., I. Arzul, B. Diggles, M. Heasman, B. Chollet, F.C.J. Berthe and M.S.J. Crane. 2006a. Development of a TaqMan PCR assay for the detection of Bonamia species. Diseases of Aquatic Organisms 71: 75-80.
Corbeil, S., I. Arzul, M. Robert, F.C.J. Berthe, N. Besnard-Cochennec and M.S.J. Crane. 2006b. Molecular characterisation of an Australian isolate of Bonamia exitiosa. Diseases of Aquatic Organisms 71: 82-85.
Dungan, C.F., R.B. Carnegie, K.M. Hill, C.B. McCollough, S.E. Laramore, C.J. Kelly, N.A. Stokes and J. Scarpa. 2012. Diseases of oysters Crassostrea ariakensis and C. virginica reared in ambient waters from the Choptank River, Maryland and the Indian River Lagoon, Florida. Diseases of Aquatic Organisms 101: 173-183.
Engelsma, M. and M. Hine. 2009. Infection with Bonamia exitiosa: disease detection, pathogen identification and typing. In: Hill, B., A. Reese, P. Dixon, B. Oidtmann, R. Paley, E. Peeler, G. Stentiford, D. Stone, K. Way, M. Hine, P. Calistri, C. Ippoliti, A. Di Lorenzo, L. Savini, O. Haenen, M. Engelsma (eds), Epidemiology of different agents causing disease in aquatic animals: scientific review and database development, (European Food Safety Authority (EFSA), Parma, Italy), Annex B, pp. 40-41.
Engelsma, M.Y., S.C. Culloty, S.A. Lynch, I. Arzul and R.B. Carnegie. 2014. Bonamia parasites: a rapidly changing perspective on a genus of important mollusc pathogens. Diseases of Aquatic Organisms 110: 5-23.
Heasman, M., B.K. Diggles, D. Hurwood, P. Mather, I. Pirozzi and S. Dworjanyn. 2004. Paving the way for continued rapid development of the flat (angasi) oyster (Ostrea angasi) farming industry in New South Wales. Final Report to the Department of Transport & Regional Services, Project No. NT002/0195 June 2004 NSW Fisheries Final Report Series No. 66. NSW Fisheries, Nelson Bay.
Hill, K.M., D.M. White, N.A. Stokes, R.B. Carnegie, N. Aloui-bejaoui, S.C. Webb, P.M. Hine, M.A. Kroeck, R. Ghars alli, R.K. Crockett, T.D. Lewis, K.S. Reece and E.M. Burreson. 2008. New perspectives on the dispersal and evolution of Bonamia species, haplosproidian parasites of oysters. Journal of Shellfish Research 27: 1016. (Abstract).
Hill, K.M., R.B. Carnegie, N. Aloui-Bejaoui, R.E. Gharsalli, D.M. White, N.A. Stokes and E.M. Burreson. 2010a. Observation of a Bonamia sp. infecting the oyster Ostrea stentina in Tunisia, and a consideration of its phylogenetic affinities. Journal of Invertebrate Pathology 103: 179–185.
Hill, B., A. Reese, P. Dixon, B. Oidtmann, R. Paley, E. Peeler, G. Stentiford, D. Stone, K. Way, M. Hine, P. Calistri, C. Ippoliti, A. Di Lorenzo, L. Savini, O. Haenen and M. Engelsma. 2010b. Epidemiology of different agents causing disease in aquatic animals: scientific review and database development (Parma, Italy, European Food Safety Authority (EFSA)), 21 p. Annex E, pp. 109-116.
Hill, K.M., N.A. Stokes, S.C. Webb, P.M. Hine, M.A. Kroeck, J.D. Moore, M.S. Morley, K.S. Reece, E.M. Burreson and R.B. Carnegie. 2014. Phylogenetics of Bonamia parasites based on small subunit and internal transcribed spacer region ribosomal DNA sequence data. Diseases of Aquatic Organisms 110: 33-54.
Hine, P.M. and J.B. Jones. 1994. Bonamia and other aquatic parasites of importance to New Zealand. New Zealand Journal of Zoology 21: 49-56.
Hine, P.M., R.B. Carnegie, M.A. Kroeck, A. Villalba, M.Y. Engelsma and B. EM. 2014. Ultrastructural comparison of Bonamia spp. (Haplosporidia) infecting ostreid oysters. Diseases of Aquatic Organisms 110: 55-63.
Kern, F.G. 1993. Shellfish health inspections of Chilean and Australian oysters. Journal of Shellfish Research 12: 366. (Abstract).
Kroeck, M.A. 2010. Gross signs and histopathology of Ostrea puelchana infected by a Bonamia exitiosa-like parasite (Haplosporidia). Diseases of Aquatic Organisms 89: 229–236.
Kroeck, M.A. and J. Montes. 2005. Occurrence of the haemocyte parasite Bonamia sp. in flat oysters Ostrea puelchana farmed in San Antonio Bay (Argentina). Diseases of Aquatic Organisms 63: 231-235.
Kroeck, M.A., L. Semenas and E.M. Morsan. 2008. Epidemiological study of Bonamia sp. in the native flat oyster, Ostrea puelchana from San Matías Gulf (NW Patagonia, Argentina). Aquaculture 276: 5–13.
Lohrmann, K.B., P.M. Hine and M. Campalans. 2009. Ultrastructure of Bonamia sp. in Ostrea chilensis in Chile. Diseases of Aquatic Organisms 85: 199–208.
Oehrens Kissner, E.M., M.S. Doldan, P.C. Zaidman, E.M. Morsan and M.A. Kroeck. 2014. Bonamiosis status in natural Ostrea puelchana beds in San Matías Gulf (Patagonia, Argentina), 14 years after an epizootic. Diseases of Aquatic Organisms 110: 135-142.
OIE Reports. 2012 & 2013. Bonamia exitiosa detected in Crassostrea virginica from North Carolina (2012) and Massachusetts (2013) with no associated disease.
Schott, E.J., J.A. Fernández-Robledo, M.R. Alavi et G.R. Vasta. 2008. Susceptibility of Crassostrea ariakensis (Fujita 1913) to Bonamia and Perkinsus spp. infections: potential for disease transmission between oyster species. Journal of Shellfish Research 27: 541-549.
White, D., N. Stokes, K. Hill, M. Kroeck, P.M. Hine, N. Aloui-bejaoui, R. Carnegie, K. Reece et E. Burreson. 2008. A molecular phylogeny of the genus Bonamia based on internal transcribed spacer region sequences. Journal of Shellfish Research 27: 1063. (Résumé).
Wilbur, A.E., J.D. Gauthier, T.D. Alphin et M.H. Posey. 2008. Preliminary investigations into the occurrence of a novel parasite (Bonamia sp.) associated with the eastern oyster Crassostrea virginica. Journal of Shellfish Research 27: 1064. (Résumé).
Citation information
Bower, S.M. (2015): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Unidentified Species of Bonamia from Various Oysters and Locations
Date last revised: February 2015
Comments to Susan Bower
- Date modified: