Haplosporidian Infection of Clams
On this page
Category
Category 1 (Not Reported in Canada)
Common, generally accepted names of the organism or disease agent
Haplosporidian infection of clams and cockles.
Scientific name or taxonomic affiliation
Little is known about the species of haplosporidians occurring in clams. Some have not been reported since the original, often brief, description. However, they seem to be correctly assigned to the phylum Haplosporidia (Burreson and Ford 2004). Also, the taxonomy of haplosporidians needs a thorough revision (Hine et al. 2009). Following is a list of parasites or reports grouped according to species and/or geographic region.
- Minchinia (=Haplosporidium) tapetis (Joly and Comps 1979, Chagot et al. 1987, Villalba and Navas 1988, Navas et al. 1992, Azevedo 2001) and plasmodia of unidentified haplosporidians (Figueras et al. 1992, Villalba et al. 1999).
- Haplosporidium edule (Carballal et al. 2001, Azevedo et al. 2003).
- Unnamed haplosporidian reported by Darriba et al. (2010).
- Unnamed haplosporidian reported by Armstrong and Armstrong (1974).
- Unnamed haplosporidian of unknown relationship to the parasites indicated above (Itoh et al. 2005).
Because some of the haplosporidians reported in clams have not been specifically identified, all reports from the same geographic region and/or species were assigned a letter code as indicated above. The information corresponding to each parasite/report was consistently assigned the same letter code in each of the following topics.
Geographic distribution
- West coasts of Portugal and Spain, and the Mediterranean coast of France.
- Galicia, Spain.
- San Martiño Island at the mouth of Ría de Vigo, Galicia, Spain. Apparently, similar parasites were reported from related species of clams along the Italian coastline.
- Yaquina Bay, Oregon, USA.
- Iwakuni in Yamaguchi Prefecture, Japan.
Host species
- Tapes (=Ruditapes) decussatus, Venerupis aureus and cultured Venerupis (=Tapes, =Ruditapes) philippinarum. Villalba et al. (1999) reported plasmodia of an unidentified haplosporidian in Venerupis rhomboides and Venerupis pullastra from Ria de Arousa, Galicia, Spain.
- Cerastoderma edule.
- Ensis arcuatus in Spain and possibly Ensis minor and Ensis sp. along the Italian coastline.
- Tresus capax.
- Venerupis (=Tapes) philippinarum.
Impact on the host
- Prevalence of infection usually low (about 4%). However, M. tapetis occurred in up to 97% of T. decussatus and 25% of V. aureus in Spain (Navas et al. 1992). The pathogenicity of the plasmodial stage in clams is minimal but the sporulation stage in the connective tissue causes important lesions in the digestive gland and gills.
- Haplosporidium edule in cockles from Spain was detected in a low prevalence at only 2 of 34 locations. Infections were heaviest in the digestive gland and induced a haemocytic response especially when the plasmodial stage was dominant (Carballal et al. 2001).
- In Spain, the intensity of infection was low and no host reaction was observed. However, in Italy, an association between the observed severe form of Haplosporidiosis and some cases of razor clam mortalities was speculated (Darriba et al. 2010).
- None reported.
- In Japan, a haplosporidian infection was detected in May 2002 in one of 40 V. philippinarum experimentally deployed in April 2002 (from Oita Prefecture, Japan) to investigate the drastic decrease in commercial V. philippinarum stocks in the area of Yamaguchi Prefecture. This is the only known case of this parasite in Japan. The gills and connective tissue of the visceral mass of the infected clam was almost completely replaced by plasmodia and spores with a few plasmodia also occurring in the foot tissues (Itoh et al. 2005).
Although no mortality events have been directly attributed to these parasites, their effect on clam populations is unknown. However, another well-known haplosporidian (Haplosporidium nelsoni) is highly pathogenic to oysters on the east coast of North America.
Diagnostic techniques
Wet Mount
- Fresh immature spores exhibit one or two (and rarely three) epispore cytoplasmic extensions. However, after 3 to 5 days of incubation in filtered seawater the extensions were lost.
- c. and d. Not described.
- Fresh mature spores examined under phase contrast microscopy had two long spore extensions (25 to 45 µm in length), one at each end of the ovoid spore.
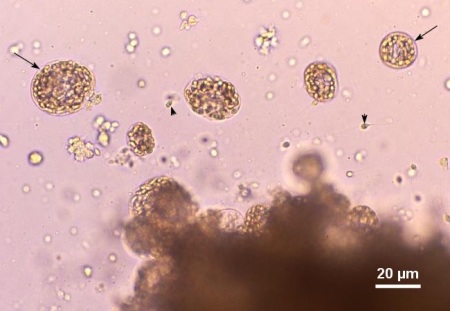
Figure 1. Wet mount squash of the digestive gland from Venerupis philippinarum from Japan heavily infected with an unidentified species of haplosporidian. Several plasmodia containing developing spores (arrows) and a few free spores (arrow heads) were liberated from the infected tissue. Image provided by Kazuo Momoyama Ph. D., Inland Sea Division, Yamaguchi Prefectural Fisheries Research Center, Aio-Futashima, Yamaguchi 754-0893, Japan.
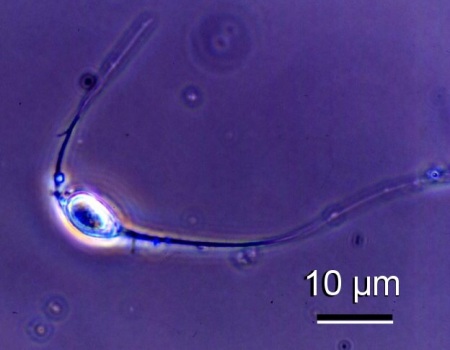
Figure 2. Phase contrast micrograph of a spore of an unidentified species of haplosporidian from Venerupis philippinarum from Japan. Image provided by Kazuo Momoyama Ph. D., Inland Sea Division, Yamaguchi Prefectural Fisheries Research Center, Aio-Futashima, Yamaguchi 754-0893, Japan.
Histology
- First signs of infection are multinucleate plasmodia usually in the epithelia of the digestive tract (Chagot et al. 1987). The plasmodia of M. tapetis and an unidentified haplosporidian from Tapes (=Ruditapes) decussatus in Galicia, Spain were usually spherical to elongated in shape with the long axis varing between 5.5 and 16 µm. These plasmodia contained 3-16 nuclei of which the larger (2 to 2.5 µm in diameter) enclosed endosomes (nucleoli) (Villalba and Navas 1988, Figueras et al. 1992). The plasmodia reported from the same species of clam in the Etang de Thau (Meditteranean coast) of France were similar in morphology (Joly and Comps 1979). Intense sporulation occurs in the interstitial connective tissue underlying the digestive gland and gills. The ovoid operculate spores are 5-6 µm long and 4-6 µm wide (Chagot et al. 1987).
- The plasmodia were mostly spherical to elongated in shape (16.72 ± 4.84 µm of length in their longest axis) and contained from two to more than 20 nuclei (Darriba et al. 2010).
- Plasmodia (5 to 6 µm in diameter), sporonts, sporoblasts (sporocysts, 11 to 15 µm in diameter) and numerous ovoid to ellipsoidal spores (3.2 by 2.2 µm) were most abundant in the connective tissue of the digestive gland but also occurred in the connective tissue of the gill, gonad and mantle (Carballal et al. 2001, Azevedo et al. 2003).
- The cysts consist of massive aggregations of host haemocytes circumscribed by fibroblast-like cells. Within the cysts, numerous oval to spherical plasmodia, ranging from 10-35 µm in length and containing 2 to more than 60 spherical nuclei. Development and multiplication of the plasmodia, and sporogony have not been observed thereby precluding positive identification.
- The connective tissue of the gills and visceral mass were almost completely replaced by parasite cells including spores. A few parasite cells were also observed in the foot tissues.
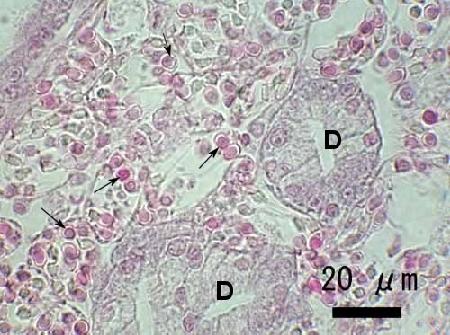
Figure 3. Heavy infection of an unidentified species of haplosporidian in the connective tissue of Venerupis philippinarum from Japan. Note the numerous developing spores (arrows) within plasmodia adjacent to the digestive gland tubules (D). Image provided by Naoki Itoh Ph. D.
Electron Microscopy
- In scanning and transmission electron microscopy, the spores were ellipsoid (about 4.8 µm long and 3.9 µm wide, n=30) with a typical hinged operculum (about 3.9 µm wide) at the apical end. The epispore cytoplasmic extensions of immature spores were formed by plasmalemma with a small amount of exosporoplasmic material (epispore cytoplasm) that lacked internal cytoskeletal structures and were not in continuity with the spore wall. The walls of mature spores had three layers (a 25 nm thick electron-dense outer layer, a 10 nm electron-light middle layer and a 15 nm thick electron-dense layer that was in contact with the endosporoplasmic membrane) and lacked ornamentation and attached cytoskeletal structures (Azevedo 2001). In addition to these structures, Hine et al. (2009) indicated that the spores also have the following ultrastructural features: microfilaments on the spore operculum, microtubules beneath the sporoplasm plasma membrane, microtubular network perpendicular to the sporoplasm membrane, exsporulation in host, cores developed in spherule-derived formative bodies which gain an outer membrane by budding to become haplosporosomes, and spores with tails that are lost with the episporoplasm. The cytoplasm of the plasmodia and spore contains ribosomes, mitochondria, haplosporosomes and organelles called spherules (Joly and Comps 1979, Chagot et al. 1987).
- Like other members of the genus Haplosporidium, the spores of H. edule had outer ornamentation composed of spore-wall material. In scanning and transmission electron microscopy, the distinctive feature of these spores is the ornamentation of slender projections (up to 1.5 µm long) with bifurcated tips as well as numerous parallel latitudinal, circumferential folds up to 0.35 µm high (Azevedo et al. 2003).
- and d. Not described.
- In transmission electron microscopy, immature spores were ovoid (3.98 ± 0.37 µm by >2.83 ± 0.18 µm, n=10) and had an apical operculum, haplosporosomes and many granules. The spore wall was comprised of three layers and no epispore cytoplasm was observed possibly because of poor sample preservation (Itoh et al. 2005).
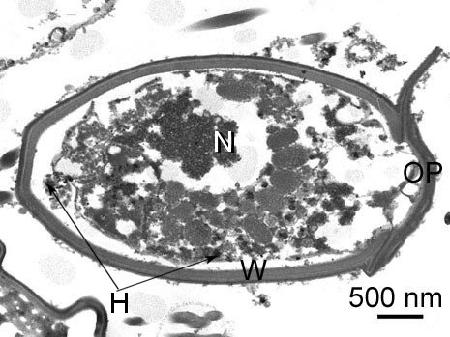
Figure 4. Section through a spore of an unidentified species of haplosporidian from Venerupis philippinarum from Japan. OP, opericulum; H, haplosporosome; N, nucleus; and w, wall of spore. Image provided by Naoki Itoh Ph. D.
Methods of control
No known methods of prevention or control.
References
Armstrong, D.A. and J.L. Armstrong. 1974. A haplosporidan infection in gaper clams, Tresus capax (Gould), from Yaquina Bay, Oregon. Proceedings of the National Shellfisheries Association 64: 68-72.
Azevedo, C. 2001. Ultrastructural description of the spore maturation stages of the clam parasite Minchinia tapetis (Vilela, 1951) (Haplosporida: Haplosporidiidae). Systematic Parasitology 49: 189-194.
Azevedo, C., R.F. Conchas and J. Montes. 2003. Description of Haplosporidium edule n. sp. (Phylum Haplosporidia), a parasite of Cerastoderma edule (Mollusca, Bivalvia) with complex spore ornamentation. European Journal of Protistology 39: 161-167.
Burreson, E.M. and S.E. Ford. 2004. A review of recent information on the Haplosporidia, with special reference to Haplosporidium nelsoni (MSX disease). Aquatic Living Resources 17: 499-517.
Carballal, M.J., D. Iglesias, J. Santamarina, B. Ferro-Soto and A. Villalba. 2001. Parasites and pathologic conditions of the cockle Cerastoderma edule populations of the coast of Galicia (NW Spain). Journal of Invertebrate Pathology 78: 87-97.
Chagot, D., Bachère, E., Ruano, F., Comps, M. and Grizel, H. 1987. Ultrastructural study of sporulated instars of a haplosporidian parasitizing the clam Ruditapes decussatus. Aquaculture 67: 262-263.
Darriba, S., D. Iglesias, M. Ruiz, R. Rodriguez and C. López. 2010. Histological survey of symbionts and other conditions in razor clam Ensis arcuatus (Jeffreys, 1865) (Pharidae) of the coast of Galicia (NW Spain). Journal of Invertebrate Pathology 104: 23-30.
Figueras, A., J.A.F. Robledo and B. Novoa. 1992. Occurrence of haplosporidian and Perkinsus-like infections in carpet-shell clams, Ruditapes decussatus (Linnaeus, 1758), of the Ria de Vigo (Galicia, NW Spain). Journal of Shellfish Research 11: 377-382.
Hine, P.M., R.B. Carnegie, E.M. Burreson and M.Y. Engelsma. 2009. Inter-relationships of haplosporidians deduced from ultrastructural studies. Diseases of Aquatic Organisms 83: 247-256.
Itoh, N., K. Momoyama and K. Ogawa. 2005. First report of three protozoan parasites (a haplosporidian, Marteilia sp. and Marteilioides sp.) from the Manila clam, Venerupis (=Ruditapes) philippinarum in Japan. Journal of Invertebrate Pathology 88: 201-206.
Joly, J.-P. and M. Comp. 1979. Etude ultrastructurale d'une haplosporidie parasite de la palourde Tapes decussatus L. (In French with English abstract) Conseil International pour l'Exploration de la Mer C.M. 1979/F:20: 5 pp.
Navas, J.I., M.C. Castillo, P. Vera and M. Ruiz-Rico. 1992. Principal parasites observed in clams, Ruditapes decussatus (L.), Ruditapes philippinarum (Adam et Reeve), Venerupis pullastra (Montagu) and Venerupis aureus (Gmelin), from the Huelva coast (S.W. Spain). Aquaculture 107: 193-199.
Villalba, A. and J.I. Navas. 1988. Occurrence of Minchinia tapetis and a Perkinsus-like parasite in cultured clams, Ruditapes decussatus and R. philippinarum, from south Atlantic coast of Spain. Preliminary results. In: F.O. Perkins and T.C. Cheng (eds.). Abstracts, Third International Colloquium on Pathology in Marine Aquaculture, 2-6 Oct. 1988, Gloucester Point, VA. Virginia Institute of Marine Science, Gloucester Point, p. 57-58.
Villalba, A., M.J. Carballal, C. López, A. Cabada, L. Corral and C. Azevedo. 1999. Branchial rickettsia-like infection associated with clam Venerupis rhomboides mortality. Diseases of Aquatic Organisms 36: 53-60.
Citation Information
Bower, S.M. (2014): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Haplosporidian Infection of Clams.
Date last revised: April 2014
Comments to Susan Bower
- Date modified: