Marteiliosis of Clams and Cockles
On this page
Category
Category 1 (Not Reported in Canada)
Common, generally accepted names of the organism or disease agent
Marteiliosis of clams.
Scientific name or taxonomic affiliation
Marteilia chriestenseni, Marteilia cochillia, Eomarteilia (=Marteilia) granula, Marteilia octospora, Marteilia tapetis, Marteilia refringens and Marteilia sp. have been reported from various species of clams and cockles in coastal waters of Europe and Asia. Marteilia spp. assigned to the phylum Paramyxea in the past (Berthe et al. 2004) were transferred to the phylum Cercozoa and order Paramyxida (Cavalier-Smith and Chao 2003, Feist et al. 2009). Tricellular spores distinguish Marteilia and Eomarteilia from other paramyxid genera. The maximum number of secondary cells (sporangial primordia) within a primary cell (sporangiosorus), in combination with the maximum number of spores within each secondary cell, is usually species-specific for Marteilia spp. (Carrasco et al. 2013). In some cases, molecular analysis was used to identify taxonomic affiliations of described species (e.g., Marteilia refringens and Marteilia sydneyi). In other cases, little is known about the species identity (e.g., an unnamed species of Marteilia-like parasite in Tridacna maxima). Itoh et al. (2014) indicated that molecular and morphological characterizations are required for the identification of unknown species. In the following text, each species of Marteilia and Marteilia-like parasite was assigned a letter code which is consistently applied to all information under each of the headings.
- Marteilia christenseni described from the clamScrobicularia plana (=piperata) by Combs 1983 (1985). The number of secondary cells in each primary cell was not indicated but each secondary cell contained 4 spores (Combs 1983 (1985)).
- Marteilia cochillia described from cockles Cerastoderma edule by Carrasco et al. (2013) and was referred to as Marteilia genotype “C” in earlier reports (Carrasco et al. 2012, Arzul et al. 2014). This parasite was initially characterized as having 4 secondary cells in each primary cell (sporangiosorus) (Carrasco et al. 2013) but Villalba et al. (2014) suggested up to 8 secondary cells in a primary cell after studying consecutive histological sections of M. cochillia. Nevertheless, there are 6 spores per secondary cell (Carrasco et al. 2013, Villalba et al. 2014).
- Eomarteilia (=Marteilia) granula described from the clam Ruditapes (=Venerupis) philippinarum (Itoh et al. 2014). Usually 8 secondary cells (sporonts) were in each primary cell (sporangiosorus) and 4 spores were contained in each secondary cell (Itoh et al. 2014). Based on molecular analysis of the 18S rDNA of Marteilia granula in comparison to other species in the paramyxean lineages, Ward et al. (2016) suggested transferring this species to a new genus, Eomarteilia to keep the genus Marteilia from being paraphyletic. This suggestion was accepted by Kerr et al. (2018) and will be used in the following text. Because the morphological characteristics of the genus Eomarteilia have not been established, E. granula retains its position on this web page as the third species to be named in the group of Paramyxida described from clams and cockles. The Marteilia sp. (possibly M. tapetis) reported by Itoh et al. (2005) in the same species of clam from Yamaguchi Prefecture, Japan had a few morphological differences from E. granula and another Marteilia sp. (probably M. tapetis) isolated from R. philippinarum in Korea had DNA that did not react with the specific primers developed for E. granula (Itoh et al. 2014).
- Marteilia octospora described from the razor shell clam, Solen marginatus by Ruiz et al. (2016). This parasite had 4 secondary cells in each primary cell (sporangiosorus) and 8 spores plus 4 nuclei were contained in each secondary cell (sporangium) (Ruiz et al. 2016).
- Marteilia tapetis described from the clam Ruditapes philippinarum (Kang et al. 2019). A large number of electron-dense striated inclusions and a small number of refringent granules occurred in the cytoplasm of the primary cell and each primary cell produced four secondary cells each of which contained four tri-cellular spores (Kang et al. 2019).
- Although Marteilia refringens is a recognized pathogen of oysters and mussels, it has been reported from several species of clams (López-Flores et al. 2008a, b; Boyer et al. 2013).
- In addition to the Marteilia spp. indicated above, unidentified species of Marteilia and Marteilia-like parasites have been reported from various other species of clams (Comps et al. 1975; Poder et al. 1983; Auffret and Poder 1987; Norton et al. 1993; Villalba et al. 1999; Ceschia et al. 2001; Berthe et al. 2004; Itoh et al. 2005, 2014; Carrasco et al. 2015; Carballa et al. 2016).
Geographic distribution
- Marteilia christenseni was described in the clam Scrobicularia plana from the area of Ronce-les-Bains, in the basin of Marennes-Oléron, on the Altantic coast of France (Combs 1983 (1985)).
- Marteilia cochillia was described from cockles Cerastoderma edule from the Ebro Delta in southern Catalonia, Spain by Carrasco et al. (2013). In 2012, M. cochillia was associated with the collapse of the C. edule fishery in Ría de Arousa, Galicia, NW Spain (Villalba et al. 2014). A Marteilia sp. was also reported in Cerastoderma (=Cardium) edule from the estuary of the Auray River, Brittany, France (Comps et al. 1975) and from oyster farming areas of north Brittany, France (Poder et al. 1983, Auffret and Poder 1987).
- Eomarteilia (=Marteilia) granula was described in the clam Ruditapes (=Venerupis) philippinarum from Odawa Bay, Miura Peninsula, Japan (Itoh et al. 2014). The Marteilia sp. detected in R. philippinarum from Iwakuni in Yamaguchi Prefecture, Japan (Itoh et al. 2005) and Korea are not the same species as E. granula (Itoh et al. 2014) and may be M. tapetis (Kang et al. 2019).
- Marteilia octospora was described from Solen marginatus from Ría de Arousa, Galicia, NW Spain (Ruiz et al. 2016) and reported from S. marginatus from North west (Galicia) (López and Darriba 2006, Ruiz et al. 2016)
- Marteilia tapetis was described in the clam Ruditapes philippinarum from three locations along the southeast coast of Korea, specifically in semi-closed bays of Goheung, Yeosu and Tongyoung (Kang et al. 2019). Possibly also from Iwakuni, Yamaguchi Prefecture, Japan where Itoh et al. (2005) detected a Marteilia sp. in R. philippinarum. (Kang et al. 2019).
- Marteilia refringens has been reported in clams as follows: Solen marginatus from Huelva on the south west coast of (Andalucía) Spain (M. refringens type M (López-Flores et al. 2008a)) and Chamelea gallina on the Balearic Islands off the Mediterranean coast of Spain (López-Flores et al. 2008b). However, in Thau lagoon on the Mediterranean coast of France, Boyer et al. (2013) reported necrotic cells of M. refringens in the digestive epithelia of some Ruditapes decussatus and suggested the non-involvement of this clam species in the parasite life cycle.
- Marteilia sp. and Marteilia-like parasites were reported in the following cockles and clams: Cerastoderma edule from the estuary of the Auray River, Brittany, France (Comps et al. 1975) and north Brittany (Poder et al. 1983); Venerupis corrugata (=Tapes pullastra) and Polititapes (=Venerupis) rhomboides from oyster rearing areas in Brittany, France (Poder et al. 1983, Auffret and Poder 1987); Polititapes (=Tapes) rhomboides from the Ensenada de Riveira and Ría de Arousa in Galicia, NW Spain (Villalba et al. 1999); Enis minor and Enis siliqua from the North east coast of Italy, Adriatic Sea (Ceschia et al. 2001, Berthe et al. 2004, Carrasco et al. 2015); Ruditapes philippinarum from Iwakuni in Yamaguchi Prefecture, Japan (Itoh et al. 2005) and Korea (Itoh et al. 2014); and Tridacna maxima from the Island of Makogai, Fiji (Norton et al. 1993).
Host species
- Marteilia christenseni was reported from Scrobicularia plana (=piperata) on the Atlantic coast of France (Combs 1983 (1985)).
- Marteilia cochillia was reported from Cerastoderma edule on the Mediterranean coast of Spain (Carrasco et al. 2011, 2012, 2013), in Ría de Arousa, Galicia, NW Spain (Villalba et al. 2014) and possibly in Cerastoderma (=Cardium) edule on the Brittany coast of France (Comps et al. 1975, Poder et al. 1983, Auffret and Poder 1987). Although plasmodia resembling immature stages of a Marteilia-like parasite were observed in the stomach epithelium of a few Cerastoderma glaucum from the Ría de Arousa, Galicia, C. glaucum was not seriously affected by M. cochillia while sympatric C. edule suffered an intense outbreak of this parasite associated with massive mortality (Carballa et al. 2016).
- Eomarteilia (=Marteilia) granula was reported in Ruditapes (=Venerupis, =Tapes) philippinarum from one location in Japan (Itoh et al. 2014). Also unidentified species of Marteilia (possibly M. tapetis) occur in R. philippinarum from Japan (Itoh et al. 2005) and Korea (Itoh et al. 2014).
- Marteilia octospora was reported in Solen marginatus from Ría de Arousa, Galicia, NW Spain (López and Darriba 2006, Ruiz et al. 2016).
- Marteilia tapetis was described in Rupitapes philippinarum from the southeast coast of Korea (Kang et al. 2019).
- In addition to oysters and mussels, Marteilia refringens was reported in Solen marginatus (López-Flores et al. 2008a), Chamelea gallina (López-Flores et al. 2008b) and necrotic-looking cells were detected in R. decussatus (Boyer et al. 2013).
- Marteilia sp. and Marteilia-like parasites have been reported in the following cockles and clams: Cerastoderma edule from Brittany, France (Comps et al. 1975, Poder et al. 1983, Auffret and Poder 1987); Venerupis corrugata (=Tapes pullastra), Polititapes (=Venerupis, =Tapes) rhomboides from Brittany, France (Poder et al. 1983, Auffret and Poder 1987); Polititapes (=Tapes) rhomboides and Ruditapes decussatus in Galicia, NW Spain (Villalba et al. 1999, Carrasco et al. 2015); Ensis minor, Ensis siliqua from the North east coast of Italy, Adriatic Sea (Ceschia et al. 2001, Berthe et al. 2004, Carrasco et al. 2015); Ruditapes philippinarum from Japan and Korea (Itoh et al. 2005, 2014); and Tridacna maxima from the Island of Makogai, Fiji (Norton et al. 1993).
Impact on the host
- Not reported. Infections in Scrobicularia plana examined by Comps (1983 (1985)) were all benign with no associated serious lesions. Since 1983, no information has been recorded on M. christenseni or its host (Carrasco et al. 2015).
- On the Mediterranean coast of Spain, a mortality event in cockles (Cerastoderma edule) during August 2008 was potentially associated with Marteilia sp. (subsequently identified as M. cochillia) where the prevalence of infection was significant (40% of 30 cockles sampled) and most had high intensities of infection (Carrasco et al. 2011, 2013). In May 2010 and January and March 2013, a Marteilia sp. (presumably M. cochillia) with about 23%, 33% and 100% prevalence respectively, was observed in C. edule from Alfacs Bay, in the Ebro Delta, during a programmed screening. Progressive C. edule mortality, starting in autumn 2012 in most of the beds, was recorded by the shellfish harvesters in the two Ebro Delta bays, and affected local commerce of C. edule in 2013. Thus, the impact of M. cochillia on the beds of cockles, in both ecological and economic terms, seems to be relevant, because it has resulted in high mortalities and economical losses (Carrasco et al. 2013). In Ría de Arousa, Galicia, NW Spain, M. cochillia was first detected in one bed of C. edule in February 2012, reached 100% prevalence by April 2012, was associated with mass mortalities on that bed by May 2012 and caused a total collapse of the fishery in the entire Ría de Arousa by September 2012 (Villalba et al. 2014). This parasite continues to cause serious problems for the cockle on natural beds and the commercial cockle fishery is highly affected (Carrasco et al. 2015). In Ría de Arousa, the cockle Cerastoderma glaucum, sympatric with C. edule in some areas, seems resistant to M. cochillia possibly due to differences in the morphology of the digestive tubules (Carballa et al. 2016). Specifically, C. glaucum has the co-occurrence of digestive tubules containing epithelial cells in different phases (i.e., holding, absorptive, digestive or excretory), and with a significant proportion of the digestive tubules in the disintegrated breakdown phase (i.e., loss of epithelial integrity with poor definition between individual cells that usually lacked nuclei and contained brownish deposits and other cell debris), whereas the digestive tubules of C. edule showed synchronicity and absence of fully disintegrated tubules. These differences could influence susceptibility to M. cochillia because the main location of this parasite in C. edule is the epithelia of the digestive gland tubules (Carballa et al. 2016). Nevertheless, Marteilia cochillia represents a notable new threat to an important European fishery species (Alfjorden et al. 2017). The microsporidian hyperparasite Hyperspora aquatic was described from primary cells of M. cochillia infecting C. edule collected in Ría de Arousa, Galicia, NW Spain (Stentiford et al. 2017).
- Eomarteilia (=Marteilia) granula was detected in 2011 and 2012 but with no remarkable mortality observed even though some of the Ruditapes philippinarum were heavily infected. A different unidentified species of Marteilia was first detected in October 2003 in the epithelium of the digestive tubules in one of 40 clams experimentally deployed in April 2002 to investigate the drastic decrease in commercial R. philippinarum stocks in the area. In both cases, no host reaction such as haemocyte infiltration, tissue degeneration nor necrosis was observed in association with the infection (Itoh et al. 2005, 2014). Because other species of Marteilia are known to be pathogenic to their respective hosts, further investigations are required before the impact of these Marteilia spp. on R. philippinarum can be determined.
- In Galicia, Spain no mortalities in Solen marginatus were associated with infection with Marteilia octospora. Surveys for parasites and disease in S. marginatus from 17 natural beds distributed along the Galician coast between 2008 and 2011 detected Marteilia sp. in 6 of them, all with low prevalence, moderate intensity and no detected negative effects on the populations of S. marginatus (Ruiz et al. 2015). López and Darriba (2006) suggested that higher prevalence and intensities of infection occurred in the summer with only heavy intensities of infection detected in August when the prevalence ranged from 38% to 42%.
- The prevalence of infection with Marteilia tapetis in clams (R. philippinarum) from south Korea was low (0.5 to 13.3%) during histopathology surveys conducted between 2009 and 2014. No pathological signs including no host tissue alterations, such as haemocyte infiltration and tissue necrosis, were observed in the tissues of infected clams (Kang et al. 2019).
- Although Marteilia refringens is a known pathogen of oysters and mussels, little information on the impact of M. refringens on clams is available (Carrasco et al. 2015). Marteilia refringens was detected in 20% (9 of 46 assayed by examining smears of the digestive gland) of Solen marginatus (López-Flores et al. 2008a). This parasite was also detected in 4.4% (3 of 69) Chamelea gallina examined during a histopathological survey conducted to discover the cause of a mass mortality in this species of clam (López-Flores et al. 2008b). In both cases, different developmental stages of M. refringens were observed in the digestive gland indicating the occurrence of an infection as seen in oysters and mussels.
- Comps et al. (1975) detected Marteilia sp. in 10% of Cerastoderma edule from one location on the south coast of Brittany, France where many cockles were moribund on the surface of the beach. Villalba et al. (1999) detected a Marteilia-like paramyxean in 7% of the seed Polititapes (=Venerupis) rhomboides (22.6 ± 1.21 mm shell length) from one location in Galicia, Spain. The low prevalence of infections by the Marteilia-like paramyxean in these clams indicated that it was not involved in the mortalities under investigation (Villalba et al. 1999). Marteilia sp. was detected in one normal looking giant clam Tridacna maxima (18 cm shell length) randomly selected from several that were collected from the wild to be used for breeding (Norton et al. 1993). The displacement of a majority of the kidney tissue by the cyst-like structures suggested that this Marteilia-like organism is potentially pathogenic to T. maxima (Norton et al. 1993).
Note that the developmental stages of these Marteilia spp. are similar to that described for M. refringens. For details see the description provided in the Marteiliosis (Aber Disease) of Oysters page and the schematic drawing that illustrates the stages.
Diagnostic techniques
Gross
a. to f. No signs of macroscopic pathology were reported.
g. At necropsy, numerous chalk-white foci were seen throughout the dark red-brown kidneys of a giant clam Tridacna maxima (Norton et al. 1993).
Histology
- Marteilia christenseni had all developmental stages characteristic of Marteilia spp. and occurred in the epithelial cells of the digestive gland tubules. The published image of a semi-thin epoxy section, stained with blue methylene borate, showed “young parasites” (likely the primary cell also called a sporangiosorus), “stages during sporogenesis” (likely early developmental stages of the secondary cell also called sporangial primordium, sporonts or sporangia), and “stages containing mature spores” (likely within mature secondary cells) (Comps 1983 (1985)).
- The developmental stages of M. cochillia in the digestive gland of C. edule were similar to those of M. refringens in oysters and mussels (Carrasco et al. 2011, 2013). Marteilia cochillia has up to eight ellipsoidal secondary cells (up to 15 µm long) in each ellipsoidal primary cell (33 µm long and 21 µm wide), and each secondary cell holds up to three proteic masses each of which contains two spores (Carrasco et al. 2013, Villalba et al. 2014). Hence, there are at most six spores per secondary cell. Darriba Couñago (2017) published images of early and late developmental stages of M. cochillia in digestive gland tubules of C. edule. As is typical of the genus, spores are tricellular and each of these cells is mononucleate. It is of note that taxonomic characters such as the number of secondary cells and spores are difficult to identify by histology, even for an experienced researcher (Carrasco et al. 2013). Comps et al. (1975) and Poder et al. (1983) indicated that the parasite that they observed in C. edule resembled Marteilia refringens in histological sections. Villalba et al. (2014) detected spores of a microsporidian-like hyperparasite in the cytoplasm of M. cochillia primary cell which also contained at least one secondary cell in which spores of M. cochillia were developing. Microsporidian hyperparasites have also been reported from the Marteilia sp. parasitizing cockles in France (Comps et al. 1975), and in M. refringens infecting Ostrea edulis in western France (Comps et al. 1979) and Mytilus galloprovincialis in Galicia, Spain (Villalba et al. 1993).
- Cross-sections of the digestive tract showed Eomarteilia (=Marteilia) granula in the epithelial cells of the stomach, digestive gland tubules and intestine of R. philippinarum. Mature secondary cells containing spores were only observed in the digestive gland tubules and free mature spores occurred in the lumen of the stomach and intestine (Itoh et al. 2014). The unique feature of internal cleavage to produce cells within cells during sporulation that differentiates Marteilia spp. from all other protista was evident in E. granula. Primary cells (sporangiosori) were 18.9 ± 2.5 µm in mean diameter (Itoh et al. 2014). The most developed primary cells contained 8 secondary cells with 4 spores in each (Itoh et al. 2014). The Marteilia sp. (possibly M. tapetis) found in the digestive gland of one R. philippinarum from Yamaguchi, western Japan, had 4 secondary cells (sporonts) containing 2 spores each within each primary cell (sporangia) (Itoh et al. 2005) and this parasite had fewer eosinophilic (“refringent”) granules than E. granula (Carrasco et al. 2015).
- The primary cells of Marteilia octospora were eosinophilic, spherical or elongate in shape and uni- or multinucleated and located in or between digestive epithelial cells of the digestive ducts and tubules of S. marginatus. More advanced developmental stages including secondary cells (sporangia) containing different stages of sporulation were also observed in these tissues and were more abundant than primary cells. The mature stage was found free in the lumen of the digestive tubules (López and Darriba 2006, Ruiz et al. 2016). For coloured images see López et al. (2011).
- Developmental stages of Marteilia tapetis were observed in the epithelial cells of the digestive tubule of R. philippinarum. Early-stage primary cells were oval in shape (4.6–14.0 μm in diameter), and mature primary cells had a small number of eosinophilic granules and contained four secondary cells, in which two to four spores were observed. Mature spores measured 11.9–17.4 μm in diameter and were occasionally observed in the lumens of the digestive tubules (Kang et al. 2019). Kang et al. (2019) also noted that all M. tapetis infected clams were co-infected with trophozoites of the protozoan parasite, Perkinsus olseni which was found mostly in the gills and mantle tissues of the clams.
- Classical developmental stages of M. refringens were found only in the epithelium of the digestive diverticula of S. marginatus (López-Flores et al. 2008a) and C. gallina (López-Flores et al. 2008b).
- Usual morphological stages of Marteilia were observed in the digestive gland epithelium of various clams infected with unidentified Marteilia sp. (Poder et al. 1983, Auffret and Poder 1987, Villalba et al. 1999, Berthe et al. 2004, Carrasco et al. 2015). However, Norton et al. (1993) reported that in histological sections, the kidneys of Tridacna maxima infected with a Marteilia-like parasite appeared as numerous cyst-like structures lined with ciliated columnar epithelium. These structures appeared to be the result of a proliferation of the ciliated epithelial lining of the ducts within the kidney. Within the cysts were groups of Marteilia-like protistan cells, the smallest were about 2 µm in diameter with dark punctate nuclei and grey cytoplasm (heamatoxylin and eosin stain). These cells were enclosed within a cellular unit which was about 4 µm in diameter and also stained grey. Further from the epithelial lining, these cells had an eosinophilic colour and indistinct outlines and contained irregular-shaped refringent inclusion bodies (Norton et al. 1993).
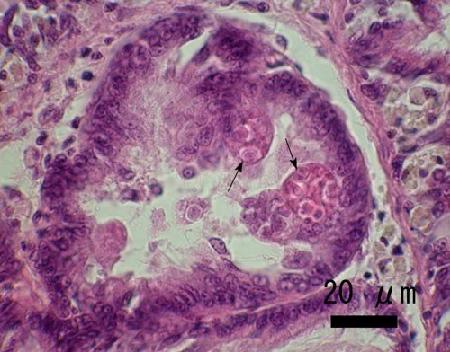
Figure 1. Mature secondary cells (arrows) containing developing spores of Marteilia sp. in the digestive gland tubule epithelium of Ruditapes (=Venerupis) philippinarum from Iwakuni in Yamaguchi Prefecture, Japan.
Tissue Imprints
Tissue imprints are a quick technique that may be a suitable for determining the number of spores per secondary cell, a feature that helps to differentiate between species of Marteilia. The procedure involves making imprints or impression smears with a piece of fresh infected tissue on a glass microscope slide followed by air drying the preparation and then acetone- (or methanol-) fixation. After the slide has dried, stain with Wright, Wright-Giemsa, May-Grunwald-Giemsa or equivalent stain (e.g., Hemacolor, Merck; Diff-QuiK, Baxter). Mounting a coverslip on the preparation will reduce light refraction making the parasites easier to see.
- Not reported for Marteilia christenseni.
- Free secondary cells (=sporangial primordium, sporonts or sporangia) of M. cochillia have finely granulated cytoplasm and contain 6 spores, each with up to 3 very small nuclei, and up to 3 extraspore nuclei (Villalba et al. 2014). Note that in geographic locations where M. cochillia was found in C. edule, Villalba et al. (2014) detected a higher prevalence of infection in histological sections than in tissue imprints.
- Itoh et al. (2014) used tissue imprints to quantify the size of secondary cells (major and minor axes of 11.2 ± 1.2 X 8.5 ± 0.8 µm), spores (5.0 ± 0.9 µm in diameter) and eosinophilic (refringent?) granules (9-18 per secondary cell each measuring 2.1 ± 0.7 µm in diameter) for E. granula. However, primary cells were ruptured by the tissue imprinting process making it necessary to use histology techniques to characterise this stage in the life cycle at the light microscope level of magnification. Mature secondary cells of E. granula contained four spores and large granules (equivalent to 'refringent' granules) that stained dark blue with Diff-QuiK.
- Tissue imprints were essential to Ruiz et al. (2016) to confirm the presence of 8 spores per secondary cell (sporangium) in M. octospora, a number never reported in other Marteilia spp. In addition to the spores, each secondary cell also contained 4 nuclei (Ruiz et al. 2016). The initial report of 6 spores per secondary cell by López and Darriba (2006) was determined from histological sections of infected S. marginatus.
- The tissue imprints of the digestive tubules of a clam (R. philippinarum) infected with Marteilia tapetis demonstrated the presence of the uninucleated early stage (primary cell), as well as other developing stages, but mature spores were not observed, possibly due to the low level of infection (Kang et al. 2019).
- Results of tissue imprints (if conducted) were not reported.
- Results of tissue imprints (if conducted) were not reported.
Electron Microscopy
- At maturity, the secondary cells of M. christenseni contain 'refringent' granules and four spores each. The spores (between 3.5 and 4.5 µm in diameter) contain haplosporosomes (90 to 200 nm in size) and have thick walls (40 to 50 nm) with complex digitations giving the profile of the spore large variability. Ultrastructural features were used to differentiate Marteilia christenseni from the four species of Marteilia described prior to 1983 (M. refringens, M. lengehi, M. sydneyi and M. maurini) (Comps 1983 (1985)). These features included the morphology of the spore (with finger-shaped wall expansions) and the size and number of refringent granules associated with the spores in the secondary cell. The table published by Comps (1983 (1985)) indicated that in M. refringens, M maurini, and M. sydneyi there were 2 to 6 refringent granules per secondary cell that ranged in size from 1.7 to 3.4 µm, the secondary cell of Marteilia sp. in C. edule from the Atlantic coast of France had about 100 refringent granules each about 0.3 µm in size while M. christenseni had about 10 to 22 refringent granules ranging in size from 0.5 to 0.8 µm.
- Early parasite stages of M. cochillia consisted of immature primary cells with haplosporosomes and striated inclusions, and containing one to four secondary cells surrounded by a membranous system. Primary cells in final stages of maturation (sporangiosorus) were ovoid (about 24 μm in length and 15 μm in width). They budded endogenously to form approximately four secondary cells (about 11 μm in length and 8 μm in width). The secondary cells (sporangial primordium, sporonts or sporangia) had a typical Marteilia spp. appearance with prominent refringent granules of potential glycoprotein nature, many ribosomes and a lack of haplosporosomes (Carrasco et al. 2013). The membrane-limited, electron-dense (refringent) granules were about 0.34 µm in diameter with up to 147 granules counted in an ultrathin section of mature secondary cells (Villalba et al. 2014). Each secondary cell also contained three and potentially four “amorphous proteic masses” (approximately 8 μm in length and 6 μm in width) which developed into spores (tertiary cells) with up to six spores per secondary cell. The spores (4.5 μm in diameter) had haplosporosomes, ribosomes and long parallel arrays of smooth endoplasmic reticulum, endogenous budding of sporoplasms (three in total: outer, intermediate and inner), and a thickened spore wall. The intermediate sporoplasm (2.7 μm in diameter) had a reniform nucleus, prominent rod-like haplosporosomes and resembled the mature sporoplasms and maturing spores of M. sydneyi (Carrasco et al. 2013).
- The secondary cell (sporont) of E. granula contained refringent granules, striated inclusions and endoplasmic reticulum. Usually eight secondary cells (sporonts) were in each primary cell (sporangiosorus) and four spores were contained in each secondary cell (Itoh et al. 2014). Like all Marteilia spp. the mature spores consisted of three cells: outermost cell with haplosporosomes and mitochondria in the cytoplasm; intermediate cell with vesicular structures in the cytoplasm and innermost cell. The mature spores were surrounded by myelin whorl remnants of the secondary cell, the inner membrane of the spore was lined with an electron dense monolayer (0.35-0.64 µm) which has not been reported from other Marteilia spp., and the nucleus of the outermost cell was degenerated (Itoh et al. 2014). The Marteilia sp. in R. philippinarum from Iwakuni in Yamaguchi Prefecture, Japan had four secondary cells (sporonts) in each primary cell (sporangiosorus) and two spores were contained in each secondary cell (Itoh et al. 2005).
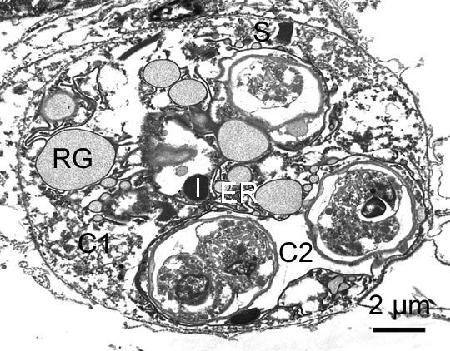
Figure 2. Secondary cell (sporont) of Marteilia sp. (possibly M. tapetis) containing developing spores from Ruditapes philippinarum from Iwakuni in Yamaguchi Prefecture, Japan. RG, refringent granules; S, striated inclusions; C1 and C2, cytoplasm of secondary cell; I, inclusion body; and ER, endoplasmic reticulum.
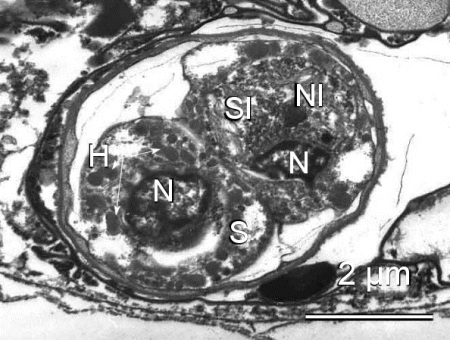
Figure 3. Spore of Marteilia sp. from Ruditapes philippinarum from Japan. Note the thickness of the spore wall, haplosporosomes (H), nuclei (N), cytoplasm of the outermost cell (S), cytoplasm of the intermediate cell (SI), and the nucleus of the innermost cell (NI).
- Ultrastructural features reported to be unique to Marteilia octospora include the size (about 0.6 µm in diameter) and number (up to 50 per ultrathin section) of electron-dense (equivalent to refringent) granules in the cytoplasm of mature secondary cells (sporangia containing mature spores), and the absence of a layer of concentric membranes surrounding the mature spore. Also, concentric membranous structures observed in different stages of the parasite have never been mentioned in other species of the genus Marteilia (Ruiz et al. 2016). Morphological details reported by López and Darriba (2006) and Ruiz et al. (2016) are as follows. Primary cells of M. octospora were uninucleated, delimited by an envelope (around 25 nm thick) and were often surrounded by numerous host mitochondria. The cytoplasm of the primary cells contained numerous ribosomes, haplosporosomes that were oblate (0.07 ± 0.01 µm long) and vermiform (0.25 ± 0.06 µm long and 0.06 ± 0.01 µm wide) in shape, paracrystalline inclusions, large spherical multivesicular bodies (2.5 ± 0.8 µm in diameter with a dark and grainy interior containing a variable number of amorphous vesicles and free linear membranes), and abundant concentric membranous structures. However, mitochondria were not observed inside the parasite cells. The cytoplasm of the secondary cell was denser than that of the primary cell and contained numerous ribosomes, haplosporosomes of similar size and shapes as those in the primary cell, multivesicular bodies were not observed and concentric membranous structures were rarely observed. The nucleus of the secondary cell was conspicuous for the presence of concentric membranous structures. However, the nuclear membrane of both the primary and secondary cells was similar, with double membrane interrupted by nuclear pores and scarce parallel membranes close to the nuclear membrane resembling endoplasmic reticulum. The size and the number of electron-dense ('refringent') granules in the secondary cells increased during maturation. In secondary cells (sporangia) with mature spores they were between 0.51 and 0.90 µm (0.65 ± 0.11 µm) in diameter with up to 50 granules counted in an ultrathin section of mature secondary cells. The mature spores (4.17 ± 0.32 µm in diameter) were surrounded by a thick envelope (41.4 ± 12.8 nm) and had 3 uninuclate sporoplasms or protoplasts (outermost, intermediate, and innermost) one inside the other. The outermost sporoplasma contained spherical haplosporosomes (95 ± 28 nm in diameter) and concentric membranous structures. The denser cytoplasm of the intermediate sporoplasma contained flattened, vermiform and double membrane-limited vesicles (220 ± 37 nm long and 54 ± 5 nm wide), numerous ribosomes, a rounded dark inclusion (195 ± 49 nm in diameter) and a nucleus. The innermost sporoplasma was the densest with numerous ribosomes and a presumed nucleus. For higher magnification of an electron micrograph of spores see López et al. (2011).
- The primary cells (mean diameter of 16.6×14.4 μm, n=3) of Marteilia tapetis contained four secondary cells, many well distributed electron-dense striated inclusions and a few refringent granules. The secondary cells (mean diameter of 8.9×7.6 μm, n=4) contained four tricellular spore cells (mean diameter of 4.6×3.8 μm, n=13). The mature spores were spherical or ellipsoid in shape and surrounded by a smooth vesicle. The cytoplasm of the outer spore cell was less dense, and appeared to have vesicular structures within the cytoplasm, although a type of flattened vermiform vesicle as reported in Marteilia octospora was not observed. An electron-dense haplosporosome-like structure was observed in the cytoplasm of the intermediate spore cell (Kang et al. 2019).
- The ultrastructure of Marteilia refringens detected in clams Solen marginatus, Chamelea gallina and Ruditapes decussatus was not examined (López-Flores et al. 2008 a, b; Boyer et al. 2013)
- The ultrastructure of Marteilia spp. reported from various clams (except those indicated above) was not reported except for Marteilia sp. found in Tridacna maxima (Norton et al. 1993). In that case, the 2-µm cells contained vermiform organelles and haplosporosomes similar to those reported in spores of Marteilia sydneyi and Marteilia refringens. The cytoplasm containing the haplosporosomes represented the outermost sporoplasm and the cytoplasm containing vermiform organelles with a double membrane structure represented the intermediate sporoplasm (Norton et al. 1993).
DNA Probes
Molecular techniques are being increasingly used primarily as confirmatory techniques of the presence of a pathogen and in disease monitoring programs. Often research focuses on the optimization of the already described techniques to gain in sensitivity and specificity of an assay and in the development of new molecular techniques (e.g., quantitative real time polymerase chain reactions (qPCR)), that are faster and easier to apply and that allow a positive diagnosis even in early stages of infection. However, as clearly indicated by Aranguren and Figueras (2016) molecular tools detect DNA sequences of the pathogen which does not imply that the pathogen is viable in the host and that the infection is established. Also, a positive polymerase chain reaction (PCR) result verifies an infection in a tested host only if the assay was properly validated for the geographic area and for the hosts examined (Burreson 2008). Consequently, molecular techniques need to be validated against other techniques, such as histology or in situ hybridization, so that the reliability of a molecular assay can be determined (Aranguren and Figueras 2016).
- Not reported for M. christenseni.
- Partial sequences of the rDNA 18S small subunit region, internal transcribed spacer-one (ITS-1) and the rDNA intergene spacer (IGS) of M. cochillia were deposited as a Marteilia sp. type “C” in GenBank (accession numbers JN820083 - JN820092 and KF314809–KF314811) (Carrasco et al. 2012, 2013). BLAST analysis of these sequences revealed maximum identities of 98%, 86% and 83% with M. refringens infecting the flat oyster Ostrea edulis and Chamelea gallina, for 18S, ITS-1 and IGS, respectively (Carrasco et al. 2012). Phylogenetic analyses provided a 100% bootstrap support to separate M. cochillia and M. refringens clusters. Differential diagnosis is possible by polymerase chain reaction- restriction fragment length polymorphism (PCR-RFLP) using the restriction enzyme Bgl II after IGS amplification as described by López-Flores et al. (2004). The enzyme cuts the 358 base pair (bp) amplicon of M. cochillia in two fragments of 192 and 166 bp whereas no digestion is observed for M. refringens (Carrasco et al. 2012, 2013). Villalba et al. (2014) designed a PCR assay for the specific diagnosis of M. cochillia. In situ hybridization (ISH) analysis can be used to confirm the presence of Marteilia spp. in the digestive gland tissues of C. edule (Carrasco et al. 2011).
- A 1784 base pair (bp) segment of the small subunit (SSU or 18S) ribosomal RNA genome was identified for E. granula (Itoh et al. 2014). BLAST search revealed that this segment had 79% and 75% identities to M. refringens from Ostrea edulis and M. chungmuensis, respectively. Further phylogenetic analysis (neighbor-joining and maximum-likelihood) clearly separated E. granula from all other known species of Marteilia (Itoh et al. 2014). Ward et al. (2016) compared the 18S rDNA of E. granula to other species in the paramyxean lineages and suggested transferring this species to a new genus, Eomarteilia to keep the genus Marteilia from being paraphyletic. A polymerase chain reaction (PCR) was designed to amplify a 260 bp product from E. granula DNA which did not amplify the DNA of other species of Marteilia. One of the probes used in this PCR and two others were designed for an in situ hybridization (ISH) assay which also did not react with the other species of Marteilia that were tested (Itoh et al. 2014).
- Molecular analysis of the ribosomal rDNA intergenic spacer (IGS) and the internal transcribed spacer (ITS-1) of M. octospora showed differences with the sequences available for other Marteilia spp. Specifically, phylogenetic analysis suggested a 84% and 86% homology with ITS-1 and IGS, respectively, with M. refringens from oysters and 82% homology with IGS of M. refringens from the same host (S. marginatus) in the south of Spain (Ruiz et al. 2016). The comparison with M. cochillia showed higher homology in ITS-1 (96%) and IGS (97%) regions but the query covered only 62% of the gene in the IGS comparison. Although López-Flores et al. (2004) considered IGS as a useful marker for differentiating species of Marteilia because of its high variability and faster sequence evolution than ITS regions of rDNA, Carrasco et al. (2015) stated that the DNA sequence database available for paramyxean parasites is still weak and requires further research. Also, the short 18S fragment available for M. octospora, although not in the most variable region of the gene, is almost identical to the corresponding region of M. cochillia (Ward et al. 2016). Thus, Ruiz et al. (2016) indicated that morphological differences were more conclusive than molecular analysis for the identification of M. octospora. Nevertheless, the specific primers based on the ITS1 region designed by Villalba et al. (2014) to detect M. cochillia did not amplify DNA extracted from S. marginatus infected with M. octospora (Ruiz et al. 2016).
- A 1741 base pair (bp) segment of the small subunit (SSU or 18S) ribosomal RNA genome (GenBank accession number AB823743) was identified for Marteilia tapetis (Kang et al. 2019). BLAST analysis revealed that the sequence of M. tapetis showed the highest similarity (89.9%) to the SSU rDNA sequences of Marteilia sydneyi (AB823742) from Sydney rock oyster in Australia and was less similar to SSU rDNA sequences of other Marteilia species: 89.6% similar to M. refringens M-type (AB889894) from Spain, 89.5% to M. cochillia (AB889895) from Spain, and 78.8% to E. (M.) granula (AB856587) from Japan. Also, phylogenetic analyses (inferred from neighbor-joining and maximum parsimony assessments) indicated that the SSU rDNA sequences of six M. tapetis isolates from three different locations in southeast Korea grouped as a single clade and that this group was genetically distinct from all described Marteilia spp. (Kang et al. 2019).
- Molecular tools described for the detection of M. refringens were used to detect this parasite in various species of clams. Specifically, analysis of the IGS rDNA product from a PCR on tissue from Solen marginatus infected with Marteilia sp. indicated between 98.2 and 99.5 % identity with M. refringens nested within the clade containing parasite strains mainly isolated from mussels (López-Flores et al. 2008a). In situ hybridization (ISH) analysis showed different developmental stages of the parasite in the digestive diverticula epithelium, which suggested a true parasitism in these individuals (López-Flores et al. 2008a). A fragment of IGS rDNA was extracted from tissues of infected Chamelea gallina embedded in a paraffin block had 99.1% identity with M. refringens isolated from O. edulis (López-Flores et al. 2008b). In situ hybridization (ISH) analysis confirmed the presence of the Marteilia sp. in the digestive gland tissues of C. gallina (López-Flores et al. 2008b). Boyer et al. (2013) detected M. refringens DNA in R. decussatus with the PCR assay for internal transcribed spacer-1 (ITS1) as described by Le Roux et al. (2001) and checked the results using the nested PCR assay targeting the rDNA intergenic spacer (IGS) according to López-Flores et al. (2004). However, in situ hybridisation showed necrotic cells of M. refringens in the digestive epithelia of some R. decussatus suggesting the non-involvement of this species in the parasite life cycle (Boyer et al. 2013).
- The molecular identity is not known for the majority of Marteilia sp. reported from clams. However, a partial sequence (1741 base pairs) of the 18S small subunit rDNA of the Marteilia sp. reported in Ruditapes philippinarum from Korea has been sequenced (GenBank accession number AB823743 initially identified as Marteilia sp. MC but now named Marteilia tapetis (Kang et al. 2019). Based on the molecular analysis of this sequence in comparison to homologous sequences from other species in the paramyxean linages, Ward et al. (2016) indicated that this parasite occurred in the same clade as other Marteilia spp. but in a different clade from Eomarteilia (=Marteilia) granula. Also, this parasite did not react with the specific primers developed for E. granula (Itoh et al. 2014).
Methods of control
There are no known methods of prevention or control. Infected clams should not be transported into areas known to be free of the disease.
References
- Alfjorden, A., M. Areskog, D. Bruno, R. Carnegie, D. Cheslett, S. Feist, S. Ford, S. Jones, A. Lillehaug, L. Madsen, T. Renault, N. Ruane and P. Vennerström. 2017. New Trends in Important Diseases Affecting the Culture of Fish and Molluscs in the ICES Area 2002 – 2015. In: Anderson, E.D., N. Ruane, R. Carnegie (eds.) ICES Cooperative Research Report No. 337, International Council for the Exploration of the Sea, Conseil International pour l'Exploration de la Mer, Copenhagen, Denmark. 50 pp.
- Aranguren, R. and A. Figueras. 2016. Moving from histopathology to molecular tools in the diagnosis of molluscs diseases of concern under EU Legislation. Frontiers in Physiology 7: Article 538, 10 pp.
- Arzul, I., B. Chollet, S. Boyer, D. Bonnet, J. Gaillard, Y. Baldi, M. Robert, J.-P. Joly, C. Garcia and M. Bouchoucha. 2014. Contribution to the understanding of the cycle of the protozoan parasite Marteilia refringens. Parasitology 141: 227-240.
- Auffret, M. and M. Poder. 1987. Pathology of the main bivalve mollusc species from oyster rearing areas in Brittany (France). Aquaculture 67: 255-257.
- Berthe, F.C.J., F. Le Roux, R.D. Adlard and A. Figueras. 2004. Marteiliosis in molluscs: A review. Aquatic Living Resources 17: 433-448.
- Boyer, S., B. Chollet, D. Bonnet and I. Arzul. 2013. New evidence for the involvement of Paracartia grani (Copepoda, Calanoida) in the life cycle of Marteilia refringens (Paramyxea). International Journal for Parasitology 43: 1089-1099.
- Burreson, E.M. 2008. Misuse of PCR assay for diagnosis of mollusc protistan infections. Diseases of Aquatic Organisms 80: 81-83.
- Carballal, M.J., D. Iglesias, S. Darriba, A. Cao, J.C. Mariño, A. Ramilo, E. No and A. Villalba. 2016. Parasites, pathological conditions and resistance to Marteilia cochillia in lagoon cockle Cerastoderma glaucum from Galicia (NW Spain). Diseases of Aquatic Organisms 122: 137-152.
- Cavalier-Smith, T. and E.E.Y. Chao. 2003. Phylogeny and Classification of Phylum Cercozoa (Protozoa). Protist 154: 341-358.
- Carrasco, N., A. Roque, K.B. Andree, C. Rodgers, B. Lacuesta and M.D. Furones. 2011. A Marteilia parasite and digestive epithelial virosis lesions observed during a common edible cockle Cerastoderma edule mortality event in the Spanish Mediterranean coast. Aquaculture 321: 197-202.
- Carrasco, N., K.B. Andree, B. Lacuesta, A. Roque, C. Rodgers and M.D. Furones. 2012. Molecular characterization of the Marteilia parasite infecting the common edible cockle Cerastoderma edule in the Spanish Mediterranean coast. A new Marteilia species affecting bivalves in Europe? Aquaculture 324-325: 20-26.
- Carrasco, N., P.M. Hine, M. Durfort, K.B. Andree, N. Malchus, B. Lacuesta, M. González, A. Roque, C. Rodgers and M.D. Furones. 2013. Marteilia cochillia sp. nov., a new Marteilia species affecting the edible cockle Cerastoderma edule in European waters. Aquaculture 412–413: 223-230.
- Carrasco, N., T.J. Green and N. Itoh. 2015. Marteilia spp. parasites in bivalves: A revision of recent studies. Journal of Invertebrate Pathology 131: 43-57.
- Ceschia, G., S. Zanchetta, M. Sello, F. Montesi, P. Antonetti and A. Figueras. 2001. Presenza di parassiti in cannolicchi (Ensis minor e Ensis siliqua) pescati nell'area costiera del Mar Tirreno meridionale e del Mar Adriatico. Presence of parasites in razor clam (Ensis minor and Ensis siliqua) harvested from coastal areas of the southern Tyrrhenian and Adriatic Seas. Bollettino Societa Italiana di Patologia Ittica 30: 20-27. (In Italian with English summary).
- Comps, M. 1983 (1985). Etude morphologique de Marteilia christenseni sp. n. parasite du lavignon Scrobicularia piperata P. (mollusque pélécypode) (Morphological study of Marteilia christenseni sp. n. parasite of Scrobicularia piperata P. (mollusc pelecypod).) Revue des Travaux de l'Institut des Pêches Maritimes 47: 99-104. (In French, with English abstract).
- Comps, M., H. Grizel, G. Tig‚ and J.L. Duthoit. 1975. Parasites nouveaux de la glande digestive des mollusques marins Mytilus edulis L. et Cardium edule L. (New parasites in the digestive gland of Mytilus edulis L. and Cardium edule L.) Comptes Rendus Académie des Sciences de Paris, Série D 281: 179-181. (In French).
- Comps, M., Y. Pichot and J.P. Deltreil. 1979. Mise en evidence d'une microsporidie parasite de Marteilia refringens agent de la maladie de la glande digestive de Ostrea edulis L. Revue des Travaux de l'Institut des Pêches Maritimes. 43: 409-412. (In French).
- Darriba Couñago, S. 2017. Atlas de Histopatoloxía, Moluscos bivalvos mariños Histopathological Atlas, Marine bivalve molluscs. Published by Intecmar. Xunta de Galicia (Consellería do Mar), Edificios administrativos - San Caetano, s/n, Santiago de Compostela, Spain. (In Spanish and English).
- Feist, S.W., P.M. Hine, K.S. Bateman, G.D. Stentiford and M. Longshaw. 2009. Paramarteilia canceri sp. n. (Cercozoa) in the European edible crab (Cancer pagurus) with a proposal for the revision of the order Paramyxida Chatton 1911. Folia Parasitologica 56: 73-85.
- Itoh, N., K. Momoyama and K. Ogawa. 2005. First report of three protozoan parasites (a haplosporidian, Marteilia sp. and Marteilioides sp.) from the Manila clam, Venerupis (=Ruditapes) philippinarum in Japan. Journal of Invertebrate Pathology 88: 201-206.
- Itoh, N., T. Yamamoto, H.-S. Kang, K.-S. Choi, T.J. Green, N. Carrasco, M. Awaji and S. Chow. 2014. A novel paramyxean parasite, Marteilia granula sp. nov. (Cercozoa), from the digestive gland of Manila clam Ruditapes philippinarum in Japan. Fish Pathology 49: 181-193.
- Kang, H.-S., N. Itoh, Y. Limpanont, H.-M. Lee, I. Whang and K.-S. Choi. 2019. A novel paramyxean parasite, Marteilia tapetis sp. nov. (Cercozoa) infecting the digestive gland of Manila clam Ruditapes philippinarum from the southeast coast of Korea. Journal of Invertebrate Pathology 163: 86-93.
- Kerr, R., G.M. Ward, G.D. Stentiford, A. Alfjorden, S. Mortensen, J.P. Bignell, S.W. Feist, A. Villalba, M.J. Carballal, A. Cao, I. Arzul, D. Ryder and D. Bass. 2018. Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions. Parasitology, Published online: 11 June 2018: pp. 1-10.
- López, C. and S. Darriba. 2006. Presence of Marteilia sp. (Paramyxea) in the razor clam Solen marginatus (Pennántt, 1777) in Galicia (NW Spain). Journal of Invertebrate Pathology 92: 109-111.
- López, C., S. Darriba, D. Iglesias, M. Ruiz and R. Rodríguez. 2011. Chapter 7: Pathology of sword razor shell (Ensis arcuatus) and grooved razor shell (Solen marginatus), In: Guerra, A., C. Lodeiros, M. Gaspar, F. da Costa (eds.) Razor clams: Biology, Aquaculture and Fisheries. Xunta de Galicia, Consellería do Mar., pp. 131-168.
- López-Flores, I., R. de la Herrán, M.A. Garrido-Ramos, J.I. Navas and M. Ruiz Rejón. 2004. The molecular diagnosis of Marteilia refringens and differentiation between Marteilia strains infecting oysters and mussels based on the rDNA IGS sequence. Parasitology 129: 411-419.
- López-Flores, I., M.A. Garrido-Ramos, R. de la Herran, C. Ruiz-Rejón, M. Ruiz-Rejón and J.I. Navas. 2008a. Identification of Marteilia refringens infecting the razor clam Solen marginatus by PCR and in situ hybridization. Molecular and Cellular Probes 22: 151-155.
- López-Flores, I., F. Robles, J.M. Valencia, A. Grau, A. Villalba, R. de la Herrán, M.A. Garrido-Ramos, C. Ruiz-Rejón, M. Ruiz-Rejón and J.I. Navas. 2008b. Detection of Marteilia refringens using nested PCR and in situ hybridisation in Chamelea gallina from the Balearic Islands (Spain). Diseases of Aquatic Organisms 82: 79-87.
- Norton, J.H., F.O. Perkins and E. Ledua. 1993. Marteilia-like infection in a giant clam, Tridacna maxima in Fiji. Journal of Invertebrate Pathology 61: 328-330.
- Poder, M., M. Auffret and G. Balouet. 1983. Etudes pathologiques et epidemiologiques des lesions parasitaires chez Ostrea edulis: Premiers resultats d'un recherche prospective comparative chez les principales especes de mollusques des zones ostreicoles de Bretagne nord (Pathological and epidemiological studies of parasitic diseases of Ostrea edulis: First results from a retrospective and comparative research of main species of molluscs in oyster farm in North Brittany.). Bases biologiques de l'aquaculture, Montpellier, 12-16 decembre 1983, IFREMER. Actes de Colloques 1: 125-138. (In French).
- Ruiz, M., S. Darriba, R. Rodríguez and C. López. 2015. Marteilia sp. and other parasites and pathological conditions in Solen marginatus populations along the Galician coast (NW Spain). Diseases of Aquatic Organisms 112: 177-184.
- Ruiz, M., C. López, R.-S. Lee, R. Rodríguez and S. Darriba. 2016. A novel paramyxean parasite, Marteilia octospora n. sp. (Cercozoa) infecting the Grooved Razor Shell clam Solen marginatus from Galicia (NW Spain). Journal of Invertebrate Pathology 135: 34-42.
- Stentiford, G.D., A. Ramilo, E. Abollo, R. Kerr, K.S. Bateman, S.W. Feist, D. Bass and A. Villalba. 2017. Hyperspora aquatica n.gn., n.sp. (Microsporidia), hyperparasitic in Marteilia cochillia (Paramyxida), is closely related to crustacean-infecting microspordian taxa. Parasitology 144: 186-199.
- Villalba, A., S.G. Mourelle, M.C. López, M.J. Carballal and C. Azevedo. 1993. Marteiliasis affecting cultured mussels Mytilus galloprovincialis of Galicia (NW Spain). I. Etiology, phases of the infection, and temporal and spatial variability in prevalence. Diseases of Aquatic Organisms 16: 61-72.
- Villalba, A., M.J. Carballal, C. López, A. Cabada, L. Corral and C. Azevedo. 1999. Branchial rickettsia-like infection associated with clam Venerupis rhomboides mortality. Diseases of Aquatic Organisms 36: 53-60.
- Villalba, A., D. Iglesias, A. Ramilo, S. Darriba, J.M. Parada, E. No, E. Abollo, J. Molares and M.J. Carballal. 2014. Cockle Cerastoderma edule fishery collapse in the Ría de Arousa (Galicia, NW Spain) associated with the protistan parasite Marteilia cochillia. Diseases of Aquatic Organisms 109: 55-80.
- Ward, G.M., M. Bennett, K. Bateman, G.D. Stentiford, R. Kerr, S.W. Feist, S.T. Williams, C. Berney and D. Bass. 2016. A new phylogeny and environmental DNA insight into paramyxids: an increasingly important but enigmatic clade of protistan parasites of marine invertebrates. International Journal of Parasitology 46: 605-619.
Citation information
Bower, S.M., Itoh, N. (2019): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Marteiliosis of clams and cockles
Contact information for co-author
Naoki Itoh, Graduate School of Agricultural Science, Tohoku University, 1-1 Amamiya-machi, Tsutsumidori, Aoba-ku, Sendai 981-8555, Miyagi Japan.
Date last revised: November 2020
Comments to Susan Bower
- Date modified: