Perkinsus qugwadi (SPX) of Scallops
On this page
Category
Category 2 (In Canada and of Regional Concern)
Common, generally accepted names of the organism or disease agent
SPX, Scallop protistan X.
Scientific name or taxonomic affiliation
Perkinsus qugwadi. Because this parasite did not enlarge in fluid thioglycollate medium nor stain blue-black with Lugol's iodine (see Culture section below), and because the DNA sequence of the ITS region of the ribosomal RNA gene is considerably different from that of other species of Perkinsus (see DNA Probes section below), Casas et al. (2002) questioned the classification of this parasite as a species of Perkinsus. Other species of Perkinsus have been described from various molluscs including scallops in Atlantic Canada and from the eastern coast of the North Pacific Ocean as well as oysters, clams and abalone.
Geographic distribution
Patchy distribution in British Columbia, Canada. To date, detected at 5 of 14 grow-out locations, specifically: Booker Lagoon, Broughton Island; Kanish Bay, Quadra Island; Read Island; Denman Island; and rarely in Cypress Bay. SPX is believed to be native to British Columbia. Infected Japanese scallop seed from Kanish Bay was inadvertently distributed to several other localities in British Columbia. It is not known if the parasite has now become established in those locations.
Host species
Patinopecten yessonesis which was introduced into British Columbia for aquaculture strictly following the International Council for Exploration of the Sea (ICES) Code of Practice on the Introductions and Transfers of Marine Organisms (Sindermann 1992, ICES 1995), and probably enzootic in other unknown native species in British Columbia.
Impact on the host
Massive proliferation of protozoan throughout the connective tissue of all organs, overwhelms the scallop and results in death. Many infected scallops have creamy-white pustules up to 5 mm in diameter on all organs but most frequently on the gonad, digestive gland and mantle. Interestingly, the extent of haemocytic response does not necessarily correspond to the level of infection. This disease can cause high mortality in juvenile scallops (often exceeding 90%) but appears to be somewhat less infective to adult P. yessoensis which usually have a lower prevalence of mortality (about 50%). However, SPX is not readily transmitted between scallops except by a low percentage (less than 15%) of heavily infected juvenile scallops (less than 40 mm shell height) in which the zoospores develop. Scallops native to enzootic areas (Chlamys rubida and Chlamys hastata) were resistant to infection and the pathogenic effects of P. qugwadi (Bower et al. 1999). Also, the application of molecular assays did not detected P. qugwadi in nine other species of bivalves from enzootic areas (Itoh et al. 2013).
The prevalence of infection decreased dramatically following 1995, and the disease was last reported in 1997, leading to speculation that the introduced P. yessonesis stocks in British Columbia, Canada had developed resistance to the disease, or that P. qugwadi had disappeared. However in October 2011, Itoh et al. (2013) found P. qugwadi and associated mortality in an aquaculture facility at one of the known locations in British Columbia where infection was detected in 44 of 100 juvenile P. yessonesis (mean ± SD shell height: 29.5 ± 4.6 mm).
Diagnostic techniques
Gross Observations
Creamy-white pustules up to 5 mm in diameter on various organs are suspect and require histological examination.
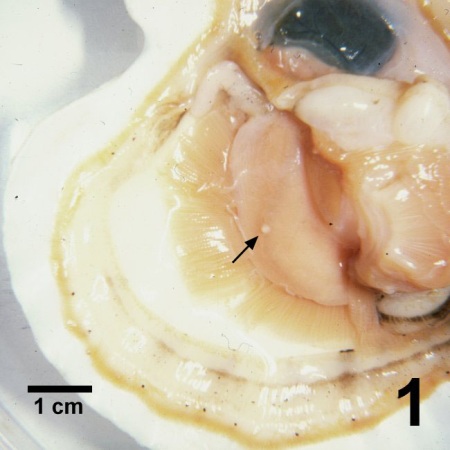
Figure 1. Patinopecten yessoensis with one valve removed showing a pustule (arrow) on the gonad caused by Perkinsus qugwadi.
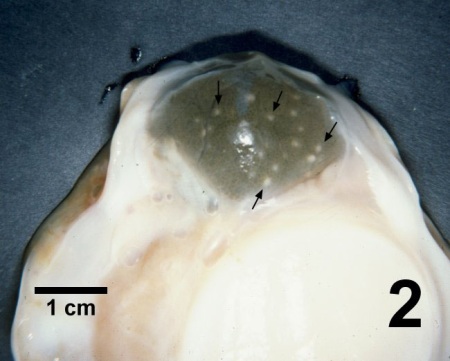
Figure 2. Digestive gland of Patinopecten yessoensis containing numerous pustules (arrows) caused by Perkinsus qugwadi.
Histology
Various organs contain round protozoa (trophozoite stage) up to 10 µm in diameter. A large vacuole may displace the centrally located nucleus in some trophozoites to form the "signet ring" stage. Tomonts consisting of clusters of 2-8 small (less than 5 µm diameter) developing trophozoites within a cell membrane may occur. Haemocyte infiltration occurs in some scallops. Zoosporangia and zoospores (tiny flagellated stage about 2-3 µm in body length) have been observed in the tissues of about 10% of the small infected scallops (less than 4 cm in shell height). The flagella of the zoospores are often not evident in histological sections but are evident in tissue imprints that are air dried and stained with most commercial formulations of Wright-Giemsa stain.
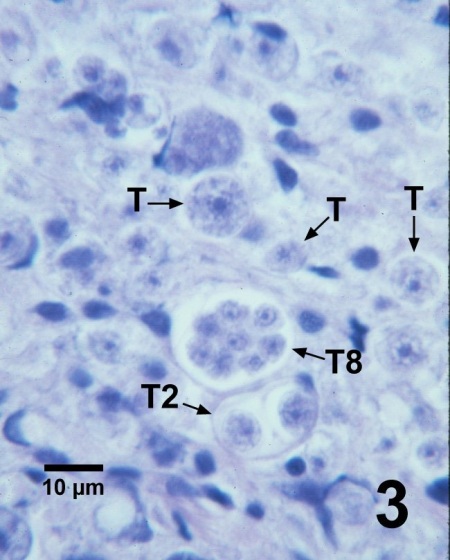
Figure 3. Histological section through the gonad of Patinopecten yessoensis with several trophozoites (T), a tomont containing two developing trophozoites (T2) and a tomont with eight developing trophozoites (T8) of Perkinsus qugwadi in the connective tissue. Haematoxylin and eosin stain.
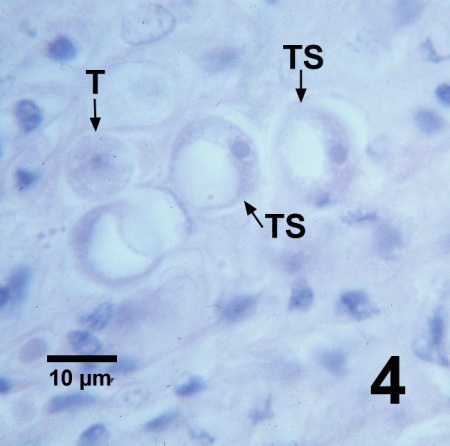
Figure 4. Histological section through the gonad of Patinopecten yessoensis containing an ordinary trophozoite (T) and a few trophozoites with large vacuoles (TS) that have displaced the nucleus to the periphery of the cell to form the "signet ring" morphology of Perkinsus qugwadi. Haematoxylin and eosin stain.
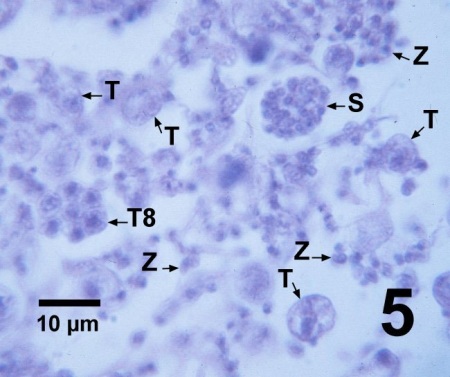
Figure 5. Histological section through a juvenile Patinopecten yessoensis heavily infected with Perkinsus qugwadi showing several small trophozoites (T), a tomont with eight developing trophozoites (T8), a zoosporangium (S) containing numerous developing zoospores, and numerous free-swimming zoospores (Z) the flagella of which are difficult to see in histology. Haematoxylin and eosin stain.
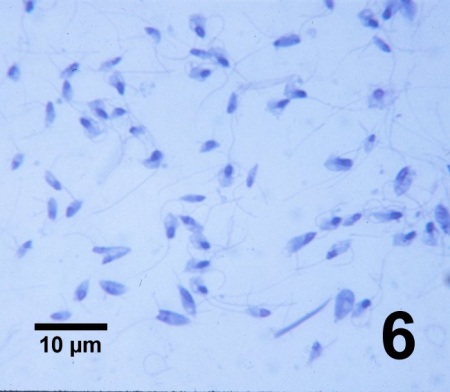
Figure 6. Biflagellated zoospores of Perkinsus qugwadi showing variable morphology in an imprint of gonadal tissue from a heavily infected juvenile Patinopecten yessoensis stained with a commercial modification of Wright-Giemsa stain.
Electron Microscopy
The ultrastructure of the zoospore is reminiscent of the zoospores of Perkinsus spp. except that the conoid associated micronemes wrap around the nucleus, some mitochondria are lobulated, an electron-dense body is lacking within the lumen of the flagella kinetosome, and the cortical alveolus-like expansions of the cell membrane occur over the entire surface of the cell. Unlike all other Perkinsus spp., the zoospores develop within the tissues of the host (scallop).
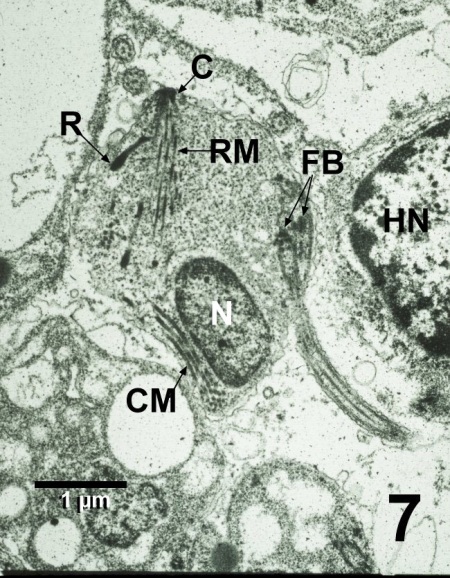
Figure 7. Transmission electron micrograph of a zoospore of Perkinsus qugwadi adjacent to the nucleus (HN) of a lysed Patinopecten yessoensis haemocyte. The section through this zoospore illustrates the bases of the flagella (FB), the nucleus (N), and the apical complex consisting of the conoid (C), rhoptry, rectilinear micronemes (RM), and conoid-associated micronemes (CM). Uranyl acetate and lead citrate stain.
DNA Probes
The sequences of segments of the ribosomal RNA gene (including parts of the small subunit (SSU rDNA or 18S rDNA) and internal transcribed spacers (ITS)) have been deposited in the GenBank database of the National Center for Biotechnology Information with accession number AF151528 and AB716689. Itoh et al. (2013) designed two PCR assays based on the ITS regions. One assay detected 52% more infections in scallops than histology; however, the assay also cross-reacted with Perkinsus honshuensis and P. olseni. The other PCR assay was less sensitive but detected 34% more positives than histology and did not react with any of the other Perkinsus species tested. These PCR assays appear to be powerful tools for screening for the presence of P. qugwadi (Itoh et al. 2013).
Phylogenetic analyses based on the ITS region of P. qugwadi consistently place this species at the base of a clade containing the other Perkinsus spp. (Coss et al. 2001; Casas et al. 2002a,b; Dungan et al. 2002). Also, phylogenetic analysis of 1796 base pairs of the SSU rRNA gene sequence clearly indicated that P. qugwadi is positioned basally to other Perkinsus species (Itoh et al. 2013).
Culture
To date, all attempts to establish P. qugwadi in cultures have been unsuccessful. In addition, P. qugwadi had no prezoosporangia development in fluid thioglycollate medium (FTM) nor subsequent dark staining with Lugol's iodine as described by Ray (1966) and used to diagnose all other known Perkinsus spp. Also, the prezoosporangia and zoosporangia that developed within the tissues of small scallops did not stain blue-black with Lugol's iodine (Blackbourn et al. 1998).
Methods of control
The extreme pathogenicity of this parasite to P. yessoensis requires that caution be taken to prevent the spread of this disease. Progeny of P. yessoensis that survived an epizootic outbreak of P. qugwadi had a significant increase in resistance to infection and resulting mortalities. Hybrid scallops, resulting from a cross between P. yessoensis females (from the same group of scallops that survived an epizootic outbreak of P. qugwadi) and Patinopecten caurinus males (native to British Columbia), had similar resistance to P. qugwadi (Bower et al. 1999). The identification of scallop stocks that are resistant to P. qugwadi has facilitated the development of a scallop culture industry in British Columbia.
References
Blackbourn, J., S.M. Bower and G.R. Meyer. 1998. Perkinsus qugwadi sp.nov. (incertae sedis), a pathogenic protozoan parasite of Japanese scallops, Patinopecten yessoensis, cultured in British Columbia, Canada. Canadian Journal of Zoology 76: 942-953.
Bower, S.M. and G.R. Meyer. 1994. Causes of mortalities among cultured Japanese scallops, Patinopecten yessoensis, in British Columbia, Canada. In: Bourne, N.F., B.L. Bunting and L.D. Townsend (eds.). Proceedings of the 9th International Pectinid Workshop, Nanaimo, B.C., Canada, April 22-27, 1993. Canadian Technical Reports of Fisheries and Aquatic Science 1994: 85-94.
Bower, S.M., J. Blackbourn, D.J.H. Nishimura and G.R. Meyer. 1990. Diseases of cultured Japanese scallops (Patinopecten yessoensis) in British Columbia, Canada. In: A. Figueras (ed.). Abstracts, Fourth International Colloquium on Pathology in Marine Aquaculture. 17-21 September 1990, Vigo (Pontevedra), Spain, Instituto de Investigaciones Marinas-CSIC, Vigo, p. 67-68.
Bower, S.M., J. Blackbourn, G.R. Meyer and D.J.H. Nishimura. 1992. Diseases of cultured Japanese scallops (Patinopecten yessoensis) in British Columbia, Canada. Aquaculture 107: 201-210.
Bower, S.M., J. Blackbourn and G.R. Meyer. 1998. Distribution, prevalence, and pathogenicity of the protozoan Perkinsus qugwadi in Japanese scallops, Patinopecten yessoensis, cultured in British Columbia, Canada. Canadian Journal of Zoology 76: 954-959.
Bower, S.M., J. Blackbourn, G.R. Meyer and D.W. Welch. 1999. Effect of Perkinsus qugwadi on various species and strains of scallops. Diseases of Aquatic Organisms 36: 143-151.
Bower, S., E. Burreson and K. Reece. 2003. Annex 10: Review of molecular techniques used to differentiate the various species/isolates of Perkinsus. Report of the Working Group on Pathology and Diseases of Marine Organisms, Aberdeen, UK, 11-15 March 2003. Mariculture Committee, ICES CM 2003/F:03, Ref. ACME, pg. 54-60.
Casas, S.M., A. Villalba and K.S. Reece. 2002. Study of perkinsosis in the carpet shell clam Tapes decussatus in Galicia (NW Spain). I. Identification of the aetiological agent and in vitro modulation of zoosporulation by temperature and salinity. Diseases of Aquatic Organisms 50: 51-65.
Casas, S.M., J.F. La Peyre, K.S. Reece, C. Azevedo and A. Villalba. 2002b. Continuous in vitro culture of the carpet shell clam Tapes decussatus protozoan parasite Perkinsus atlanticus. Diseases of Aquatic Organisms 52: 217-231.
Coss, C.A., J.A.F. Robledo and G.R. Vasta. 2001. Fine structure of clonally propagated in vitro life stages of a Perkinsus sp. isolated from the baltic clam Macoma balthica. The Journal of Eukaryotic Microbiology 48: 38-51.
Dungan, C.F., R.M. Hamilton, K.L. Hudson, C.B. McCollough and K.S. Reece. 2002. Two epizootic diseases in Chesapeake Bay commercial clams, Mya arenaria and Tagelus pledius. Diseases of Aquatic Organisms 50: 67-78.
ICES (International Council for the Exploration of the Sea). 1995. ICES Code of Practice on the Introductions and Transfers of Marine Organisms 1994. International Council for the Exploration of the Sea, Copenhagen.
Itoh, N., G.R. Meyer, A. Tabata, G. Lowe, C.L. Abbott and S.C. Johnson. 2013. Rediscovery of the Yesso scallop pathogen Perkinsus qugwadi in Canada, and development of PCR tests. Diseases of Aquatic Organisms 104: 83-91.
Sindermann, C.J. 1992. Role of the International Council for the Exploration of the Sea (ICES) concerning introductions of marine organisms, In: Rosenfield, A., R. Mann (eds.) Dispersal of Living Organisms into Aquatic Ecosystems. Maryland Sea Grant College, College Park, pp. 367-376.
Citation Information
Bower, S.M. (2013): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Perkinsus qugwadi (SPX) of Scallops.
Date last revised: May 2013
Comments to Susan Bower
- Date modified: